All Issues
Transgenic Bt crops and resistance: Broadscale use of pest-killing plants to be true test
Publication Information
California Agriculture 52(6):14-20. https://doi.org/10.3733/ca.v052n06p14
Published November 01, 1998
PDF | Citation | Permissions
Abstract
More than 10 million acres of transgenic insect-resistant crops, including cotton, corn and potatoes, were planted in the United States in 1998 — and growers are on the verge of much more extensive plantings. Genetically engineered to produce insecticidal proteins of the bacterium Bacillus thuringiensis, these plants provide effective, environmentally safe pest control. However, current transgenic crops may lead to insect resistance, partly because they have been engineered to produce only single Bt insecticidal proteins, and partly because plant senescence can result in lower production of Bt proteins as crop plants age. Australia cotton growers, for instance, found they had good control for the first half of the season in 1997, but required insecticide treatments in the latter half. Resistance avoidance strategies and crop varieties in the pipeline that produce two or more insecticidal proteins are planned to provide long-term resistance management. This is crucial not only to growers using the transformed crops, but to organic growers who rely on traditional Bt insecticides. If successful, this new technology promises high crop yields as well as benefits to most nontarget arthropods and biological control insects by reducing the use of broad-spectrum chemical insecticides.
Full text
In the 1998 growing season, U.S. growers planted approximately 9 million acres of transgenic corn and 2.8 million acres of transgenic cotton, genetically engineered to produce insecticidal proteins of the bacterium Bacillus thuringiensis (Bt). This acreage is expected to grow to about 30 million acres of corn and 6 million acres of cotton within 5 years, representing, respectively, about one-third of the corn and one-half of the cotton acreage in the United States. Bt crops offer advantages over conventional crops in that the insecticidal proteins are produced by the plants on a continuous basis, reducing the material and application costs of using a synthetic chemical insecticide. Growers have reported improved profit margins averaging $30 to $60 per acre during the first few years of Bt-cotton plantings in the Southeast. About 40,000 acres of Bt-potatoes are grown in the United States and Canada, and demand is increasing.
Transgenic Bt Crops
In 1981, scientists cloned the first Bt toxin gene, which led quickly to the development of the first transgenic Bt plants in the mid-1980s. Since then, most major crops that suffer substantial economic damage from caterpillar and beetle pests have been genetically engineered to produce Bt toxins to control these insect pests, though only a few of these are available commercially. In the United States, the available Bt-transgenic crops are cotton, corn and potatoes. Numerous others under development include rice, soybeans, broccoli, lettuce, walnuts, apples and alfalfa. The largest U.S. plantings so far have been in the Midwest with Bt-corn from Monsanto and Novartis, and Monsanto's Bt-cotton (Bollgard) in the Southeast. Within the next few years, however, these and other companies plan to plant millions more acres of Bt-corn, and plantings of Bt-potatoes are likely to grow to tens of thousands of acres. Moreover, if these crops prove to be economic successes, many others, including minor crops, will eventually be engineered to produce Bt toxins to control their major insect pests.
Resistance development concerns
While Bt crops would appear to be on the verge of major agronomic successes in several crop systems, minor failures have occurred in the first three growing seasons (1996–1998). In the United States, corn earworm (Helicoverpa zea) populations invaded limited acreages of Bt-cotton in Texas, virtually destroying the crop due to their high tolerance to the Bt toxin (Cry1Ac) produced by transgenic plants. In 1998, in Australia, where the principal cotton pests are bollworms (H. armigera and H. punctigera), which are also quite tolerant to this toxin, the efficacy of the Bt-cotton lasted for only one-half the season. Applications of other pesticides, including Bt-based insecticides, were needed to control these pests.
The lack of control resulted not only from the lower sensitivity of the bollworms to the toxins, but because the levels of the toxin (Cry1Ac) decreased gradually in the cotton plants over the growing season. One problem with such decreasing toxin levels is that this could prime target populations for the development of resistance to the toxin used in the plants and to other related Bt toxins to which these populations may initially be quite sensitive. This is because low doses of toxin eliminate the most sensitive insects, leaving a more toxin-tolerant population, in which resistance can develop faster. Moreover, because most Bt toxins have a similar mode of action, once resistance develops to one toxin, it can confer resistance to other related toxins. This is known as “cross-resistance” and has already been demonstrated in laboratory studies of Bt crystalline toxins (Cry toxins).
One reason resistance could develop is that the transgenic cotton and corn varieties currently marketed each produce only one toxic Bt protein, compared to the principal Bt strain used in insecticides to control caterpillars, which contains four major toxins in addition to other factors that increase insect mortality. Although early successes of the transgenic cotton and corn are impressive, resistance strategies will become increasingly important as total acreage expands.
Were resistance to occur, the value of microbial insecticides based on Bt proteins could be greatly diminished owing to the target insect's lower sensitivity to the Cry proteins. The environmental impact could be serious as well, because farmers would have to return to the use of broad spectrum chemical insecticides. Organic farmers, who rely even more heavily on Bt insecticides, could be severely affected.
The potential for the development of resistance to Bt proteins is not only of concern to environmentalists, but to most scientists in academia, government and the Bt insecticide and transgenic plant industry. With respect to the latter, failure of Bt-crops in the field after more than a decade of high development costs would result in significant financial losses to these companies. Monsanto has estimated that it invested $30 million to $50 million in 10 years of testing and development of Bt-resistant cotton.
Other concerns involve the safety of Bt transgenic crops to non-target organisms. Safety studies have demonstrated that vertebrate stomach juices rapidly inactivate Bt proteins. Most Bt proteins are insect-specific and become active only in the insect gut, which in most target insects is alkaline, rather than acidic as in most vertebrates including humans. Despite their general safety, Bt transgenic plants as well as the bacteria from which the protein genes are derived have some harmful effects on nontarget insect species. These effects are much less than the effects of chemical pesticides.
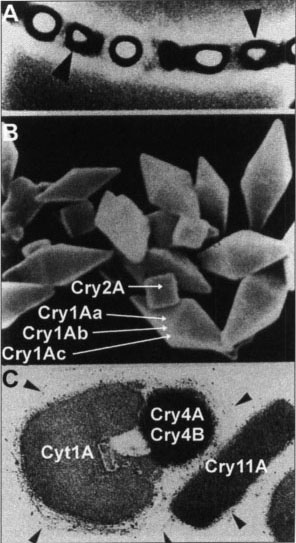
Fig. 1. Sporulated culture and parsporal bodies of Bacillus thuringiensis. A. Sporulated culture of Bacillus thuringiensis illustrating the spore and toxin-containing parasporal body. B. Parasporal body protein inclusions containing Cry proteins produced by the HD1 isolate of B.t. kurstaki, the Bt isolate used most widely in products for control of lepidopterous pests. C. Protein inclusion characteristic of B.t. israelensis used widely to control the larvae of mosquitoes and blackflies.
Biology of a success story
Understanding the reasons for resistance, and placing these concerns in perspective, requires knowledge of Bt's basic biology, mode of insecticidal action and the Bt products used as insecticides.
Insecticides based on the bacterium, Bacillus thuringiensis, have been used in many regions of the world for more than 30 years to suppress numerous lepidopteran pests of forests, vegetables and field crops, and more recently to control beetle pests and the larvae of many species of vector and nuisance mosquitoes and blackflies. Bt's success is due to the high efficacy of its insecticidal proteins, the existence of a diversity of proteins that are effective against a range of important pests, its relative safety to nontarget insect predators and parasites, its ease of mass production at relatively low cost, and its adaptability to conventional formulation and application technology.
Bt consists of a complex of more than 50 spore-forming bacterial subspecies found commonly in soil, grain dust, on plants and in insects. Under field conditions, the subspecies used in commercial bacterial insecticides typically have a restricted insect spectrum, being toxic to the larvae of either lepidopterous insects (moths and butterflies), dipterous insects (flies and mosquitoes), or coleopterous insects (beetles). The insect spectrum and specific toxicity of a subspecies is due to one or more insecticidal proteins, referred to as delta-endotoxins (δ-endotoxins), produced during sporulation and assembled into a crystalline parasporal body (fig. 1). The δ-endotoxins fall into two main classes, crystalline proteins known as “Cry” proteins, and cytotoxic proteins known as “Cyt” proteins (table 1).
A field of Monsanto's Bollgard cotton with a border of non-transgenic cotton. The Bt-transgenic cotton synthesizes the Cry1Ac protein aimed principally at the control of tobacco budworm, Heliothis virescens, the major cotton pest throughout much of the Southeast. Inset, Bt cotton and cotton from an untreated control plot.
More than 60 different Cry proteins and 4 Cyt proteins have been isolated over the past 20 years. All are encoded by genes carried on plasmids, circular pieces of DNA that can be transmitted from one Bt subspecies to another. The transmissibility of these plasmids, and mutations arising during evolution, are responsible for the widespread occurrence and diversity of Bt proteins that occur naturally in the environment. The role of these proteins is to kill certain insects, providing Bt with a rich resource in which the spore can germinate, grow and reproduce.
Bt's mode of insecticidal action
Unlike most synthetic chemical insecticides, Bt toxins are not contact poisons; they must be eaten to be toxic. When a sensitive insect such as a caterpillar ingests the bacterium with its complex of toxins, they dissolve upon encountering the alkaline (pH 8–10) juices of the midgut. Most Cry toxins contain an active “core” about half the size of the total toxin; the core is released in the midgut by digestive cleavage of the molecule. These activated toxin molecules bind to specific receptors on the surface of the insect's gut. Binding is an essential step of insecticidal action; in susceptible insects the toxicity of a particular Bt protein is correlated with the number of receptors on stomach cells. After binding, it is thought that the toxin molecules insert into the gut membrane and form pores. These pores cause leakage and swelling of the cells due to an influx of positive ions and then water. The swelling continues until the cells lyse, allowing the alkaline gut juices to leak into the insect's blood, raising the blood pH, which causes paralysis and insect death.
Cyt proteins are about one-fifth the size of Cry proteins and are unrelated chemically. However they dissolve and are processed like Cry proteins, releasing a toxic core which also causes lysis of gut cells. They differ from Cry proteins in their mode of action in that they attack the lipid portion of the cell membrane, causing lysis by either acting as detergents or by forming pores. In addition to being toxic, Cyt proteins increase the toxicity of the Cr) 4 and Cry 11 proteins, with which they occur, to mosquitoes and blackflies.
Bt-based bacterial insecticides
More than 30 Bt formulations are on the market in the United States, and most of these are complex mixtures based on sporulated cells containing the spores and array of toxins found in either B.t. kurstaki, which is used to control a wide range of moth pests, or B.t. israelensis, used to control the larvae of numerous mosquito and blackfly species. To produce the insecticides, companies grow up the bacteria, then harvest the spores and parasporal bodies, the latter which are crystalline inclusions that contain the toxins. Both subspecies owe their success to their broad spectrum of activity and the absence of any widespread insect resistance after many years of use. These characteristics are due to the composition of the specific Bt bacterial strain used in the products, especially to the toxin complexity of the parasporal body, which in each case contains four major proteins, and to the moderate frequency of use.
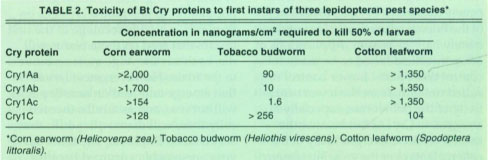
The first commercial formulations of Bt marketed over 30 years ago, such as Dipel and Thuricide, and still commonly used today, are those based on the HD1 isolate of B.t. kurstaki. The parasporal body of HD1 consists of four Cry proteins, Cry1Aa, Cry1Ab, Cry1Ac, and Cry2A (fig. 1). These vary in their insect spectrum and specific toxicity (table 2), and in combination give this isolate a broad spectrum of activity against a wide range of caterpillar species attacking field (cotton, corn, soybeans), vegetable (tomatoes, broccoli, lettuce, cabbage), fruit (strawberries, grapes, peaches) and forests (deciduous and fir trees). This protein complexity probably also accounts for the lack of economically important resistance after more than 30 years of use in all but a very few species of lepidopterans, the notable exception being larvae of the diamondback moth, P. xylostella.
Toxin complexity can delay resistance because there is usually at least one different receptor for each of the Cry proteins, even though some can share the same receptor. Whereas insects in a target population are unlikely to be sensitive to all four Cry proteins, moderate to high sensitivity to two or three reduces the probability that a substantial number of insects within the population will be resistant to all toxins. Under conditions of moderate Bt usage, that is, low selection pressure, the frequency of individuals containing a full set of resistance genes remains low, which translates into little or no Bt resistance.
In addition to the parasporal body toxins, B.t. kurstaki and many Bts produce several other components that improve the efficacy of the Cry proteins, increasing its insect spectrum, and impeding resistance. These include the antibiotic zwittermicin, which increases the toxicity of Cry proteins by an unknown mechanism, and the spore. The spore has its greatest effect against insects with moderate or low sensitivity to the Cry toxins, such as larvae of gypsy moth (Lymantria dispar), the diamondback moth (Plutella xylostella) and the beet armyworm (Spodoptera exigua). In larvae of these species, after an initial intoxication resulting from activation of the Cry toxins in the midgut, the spore germinates and produces enzymes (phospholipases and proteases). These contribute to the lysis of gut cells by degrading cell membranes.
With respect to B.t. israelensis, strains derived from the ONR60A isolate used in commercial products are the most successful. The parasporal body of this isolate is highly toxic to mature larvae of Aedes and Culex mosquitoes as well as to the larvae of numerous species of blackflies. This high toxicity and broad spectrum among mosquitoes, blackflies, and related dipterans are due a complex of four major proteins, Cry4A, Cry4B, Cry11A and Cyt1A (fig. 1). Aside from the number of toxins, the Cry and Cyt proteins of B.t. israelensis contribute to its effectiveness in two ways. First, combinations of Cyt and Cry proteins as well as combinations of Cry proteins interact synergistically increasing the toxicity of each other from three-to fivefold.
Second, laboratory studies suggest that the Cyt1A protein delays the development of resistance to the Cry4 and Cry11A proteins. In experiments with larvae of the mosquito Cx. quinquefasciatus, toxin combinations containing Cyt1A led to only a 3.2-fold level of resistance after 28 generations, whereas high levels of resistance, from 90 to greater than 100-fold, were obtained in combinations of Cry toxins lacking Cyt1A. The mechanism by which the CytA protein enhances toxicity and delays resistance is not known. In the field, products based on B.t. israelensis have been in use for well over a decade, yet no resistance has reported in mosquito or blackfly populations. This lack of resistance may be due not only to the complex protein composition of the B.t. israelensis parasporal body, but to treatment of only limited breeding areas, which permits seasonal and year-to-year mixing of treated and untreated mosquito populations, thereby reducing the buildup of resistance genes in the treated populations.
Concerns about Bt transgenics
All first generation transgenic Bt-crops are based on plants that produce only a single Bt protein, lacking the complexity of conventional Bt-based bacterial insecticides. For example, current lines of Bt-cotton produce the Cry1Ac protein, and are targeted to control the tobacco budworm, the most important cotton pest in the southeastern United States. Cry1Ac was selected for engineering into cotton because it is the most toxic to the tobacco budworm (table 2). Similarly, in the case of corn, most lines have been engineered to produce only the Cry1Ab toxin to control the European cornborer (Ostrinia nubilalis), and in potatoes to produce only the Cry3A toxin to control the Colorado potato beetle.
In addition to the lack of complexity in comparison to bacterial insecticides, each of these toxins is produced continuously by the plant. While perhaps an advantage from the standpoint of saving application costs, continuous toxin production places the insect population under heavy selection pressure. During the past two growing seasons, studies of Bt cotton in the United States and Australia have shown that Cry1Ac production is continuous, but can decrease over the growing season, in some cases leading to sublethal doses for budworm larvae near the end of the season. This was the reason that growers in Australia experienced control problems with their caterpillar pests. Aside from problems during a particular growing season, as noted above, sublethal doses can prime populations for resistance because it is the most tolerant individuals in the population that survive.
Because first generation Bt crops generally target only a single pest species, another problem they present is lack of adequate control of insects not very sensitive to the toxin produced by the crop. It is important to realize that lack of or low sensitivity is not resistance. Resistance is defined as a statistically demonstrated decrease in sensitivity to a toxin by a population in response to use of the toxin as a killing agent. If there is little or no sensitivity to begin with, this cannot be resistance, but nevertheless can lead to control problems. For example, as can be seen from table 2, the Cry1Ac protein is not very toxic to species of armyworms (Spodoptera species), or boll-worms (Helicoverpa species). Already problems have been encountered in the United States and Australia in Bt-cotton with bollworms. In Texas, high corn earworm (Helicoverpa zea) populations invaded limited acreages of Bt-cotton, virtually destroying the crop due to their high tolerance to Cry1Ac. In 1997 in Australia, where the principal cotton pests are bollworms (H. armigera and H. punctigera), which are also moderately tolerant to Cry1Ac, the efficacy of the Bt-cotton lasted only for approximately half the season. Applications of other insecticides had to be made to control these pests. Lower control resulted not only from the lower sensitivity of the bollworms, especially H. armigera, to Cry1Ac but because the toxin level decreased gradually in the cotton plants over the growing season.
Bt resistance management
The possibility of resistance to transgenic crops has prompted the development of a variety of conceptual strategies for managing resistance. The most prominent of these are listed in table 3 (McGaughey and Whalen 1992; Tabashnik 1994; Gould 1998). Most of these, for example, mixtures of various toxin genes within plants, the use of tissue-specific toxin production, and induced toxin synthesis in which toxin is only synthesized after an insect begins to feed, are years away from field deployment or commercial availability. In mixing genes within a crop plant (also known as pyramiding or stacking genes), genes for two or more insecticidal proteins, of the same or different types, are engineered into the same plant. In tissue-specific expression, the plants are engineered so that the toxin gene is introduced into the plant in such a way that it is only produced in tissues on which the insect feeds, for example, depending on the target insect, only in the roots, or only in the leaves. In the induced toxin synthesis strategy, the gene only produces the toxin protein after insect feeding is initiated. The molecular tools for developing such plants are already available.
In the meantime, resistance management relies primarily on using a high dose and refuge strategy. In this strategy, some percentage of the crop, usually 4% to 20%, must consist of non-Bt plants. And the non-Bt plants must be planted together, usually as strips along the edge of the crop, or as blocks within the crop.
The value of the non-Bt plants is to maintain a high percentage of susceptible insects, that is, a high frequency of susceptible genes, in the target population. When moths lay eggs on Bt-plants, a high percentage of the first instars that feed on these plants will die, as this is the stage most sensitive to the toxin. However, most larvae that emerge and feed on non-Bt plants will survive, and as adults, theoretically these will mate with adults that survived on Bt-plants. The latter survivors presumably survived because they were heterozygous or homozygous for resistance. They should constitute a low percentage of the mating population, and by mating with insects not selected for resistance, the percentage of resistance genes is diluted and remains low in the target population.
The high dose/refuge strategy is being used to manage resistance in Bt-cotton, Bt-corn and Bt-potatoes in the United States. Growers have a contractual obligation to the companies, mandated by the U.S. Environmental Protection Agency, to implement the refuge strategy, and use refuges of a specified size. Two types of refuges are possible, small refuges that are not allowed to be sprayed with any insecticides, and larger refuges that may be sprayed. For example, in Bt-corn, the unsprayed refuge is 4%, and the sprayed refuge 25%. In Bt-corn, the unsprayed refuge is 20%, and the sprayed refuge 30% to 50%. Spraying the refuges with a chemical insecticide, of course, will kill the target pest and therefore reduce the population of insects sensitive to the toxin. However, the rationale underlying this strategy is that only about 80% of the insects treated with chemical insecticides will be killed, and thus a sufficient refuge population of sensitive insects will remain to delay or avoid resistance. The size of the refuges are based on models, and remain to be validated under field conditions.
Bt-cotton has been planted for 3 years, but it is not yet possible to fully assess its success. During the first 3 years, there has been no confirmed evidence of resistance. But aside from the refuges planted in the crops, many surrounding non Bt-crops and noncrop plants provide refuges for susceptible insects, which then contribute to the dilution of resistance genes in the target population gene pool. The true test of this strategy will come when large contiguous areas, comprising square miles of Bt-crops producing Cry genes, are planted successively for several years.
While these experiments in nature are ongoing, more sophisticated plant engineering strategies are being used to engineer resistance management strategies directly into the plants. In addition to using combinations of Cry genes in the same crop plant, mixtures of insecticidal proteins with different modes of action are under development. Already several types of proteins to meet these needs have been identified, including non-Cry proteins from Bt, insecticidal non-Bt toxins, lectins, and enzymes selectively toxic to specific insects.
Resistance to Bt insecticides
Though resistance to Bt products under field conditions has been rare, laboratory studies show that insects are capable of developing high levels of resistance to one or more Cry proteins. Under laboratory selection, for example, populations of the indianmeal moth (Plodia interpunctella) developed levels of resistance ranging from 75- to 250-fold to Cry1Aa, Cry1Ab, Cry1Ac, Cry2A, and Cry1C (Tabashnik 1994; Gould 1998). In addition, under heavy selection pressure in the laboratory, populations of mosquitoes (Cx. quinquefasciatus), beetles (the Colorado potato beetle and the cottonwood leafbeetle, Chrysomela scripta), and the tobacco budworm (Heliothis virescens), all developed levels of resistance ranging from several hundred to several thousandfold to the Cry toxins against which they were selected (Bauer 1995; Georghiou and Wirth 1997). Rotation of Cry proteins in bacterial insecticides is a potentially useful tactic for managing resistance to individual Cry proteins. However, because most Cry proteins are related, the potential for cross-resistance remains a major problem. In fact, high levels of cross-resistance among Cry proteins has already been demonstrated in laboratory populations of the tobacco budworm (Gould et al. 1995).
Above, Bt-transgenic corn to the left of a field of nontransgenic corn. Most Bt-transgenic corn developed to date produces the Cry1Ab protein for control of the European cornborer, Ostrinia nubilalis. Below, Comparison of a Bt-transgenic corn stalk (top) with a cornstalk damaged by the European cornborer.
Whereas the above results were obtained using laboratory models, resistance in the field has become a major problem in the diamondback moth in Hawaii, Japan, the Philippines and Florida, where populations were treated heavily and frequently, weekly or more, with products based on the HD1 isolate of B.t. kurstaki. In Hawaiian populations, owing to cross-resistance, resistance extended to include Cry1Aa, Cry1Ab, Cry1Ac, Cry1F and Cry1J, whereas certain Floridian populations were resistant to Cry1Aa, Cry1Ab, and Cry1Ac. These results make it clear that even with complex products containing mixtures of Cry endotoxins and synergists, resistance in the field is a significant threat when pest populations are placed under intensive selection pressure.
Other concerns about Bt-crops
Concerns have also been raised about the safety of Bt proteins, and therefore Bt-crops, to nontarget insects, especially the predators and parasites used as biological control agents. The purpose of Bt, of course, is to kill the target pest, but even Bt insecticides also cause mortality in certain nontarget insects. For example, Bt products used to control caterpillar pests such as gypsy moth and spruce budworm larvae in forests will also kill certain species of nontarget lepidopterous larvae in these habitats.
The mortality caused in the nontarget populations should be kept in perspective and viewed in the context of the relative risk of using Bt in comparison to using available synthetic chemical insecticides. The latter typically have a broader spectrum of toxicity, and will kill pest and nontarget insects belonging to a wide range of insect orders. Because all Bt proteins have a very restricted spectrum of activity under field conditions, the use of Bt proteins is much more environmentally compatible than the use of chemical insecticides. Bt proteins may persist in the environment, when used in either insecticides or Bt-crops, but their toxic effects on nontarget predators and parasites is low and temporary, being reduced even further after the crop is harvested.
The Bt-transgenic potatoes — in the middle of two rows of nontransgenic potatoes — produce the Cry3A protein, which protects them from damage by the Colorado potato beetle, Leptinotarsa decemlineata.
It should also be obvious that as a control agent targeted to kill insect pests, Bt-crops will also reduce the predator and parasite populations, particularly the latter, that depend on the target insect for their reproduction. Again, the mortality caused by Bt in these nontarget populations must be kept in perspective. Most crop production, especially that of field, vegetable and fruit crops, occurs in monocultures that are not natural ecological habitats. The crop uniformity characteristic of these monocultures permits pest insects as well as their predators and parasites to build up into populations that are much larger than those that would occur in more diverse natural habitats. In this context, the use of Bt-crops has a neutral effect in that it reduces predators and parasite populations that would not occur if it were not for the unnatural presence of a large crop habitat and concomitantly large host pest populations that the predators and parasites use as a resource. Moreover, even in the unnatural ecological habitat of a crop monoculture, the use of Bt, owing to its greater specificity, will have less impact overall on the predator and parasite populations than will the use of broad spectrum chemical insecticides.
Conclusion
Bt-crops provide an effective and environmentally safe alternative to conventional pest control methods. It is easy at present to be critical of first generation Bt-crops, as they represent simple constructs that could fail. However, with any new technology, there are likely to be problems. More importantly, tools and concepts have already emerged for making better second and third generation transgenic insecticidal crops, and this trend should continue. It is important to keep in mind that transgenic crops have the potential not only of being better from an agronomic perspective, but as they greatly reduce the need for synthetic chemical insecticides, they are much better for the environment, especially nontarget organisms, including the predators and parasites used in biological control.