All Issues
Phytophthora ramorum can survive introduction into finished compost
Publication Information
California Agriculture 69(4):237-241. https://doi.org/10.3733/ca.v069n04p237
Published online October 01, 2015
NALT Keywords
Abstract
Composted municipal green waste is a potential vehicle for the transmission of Phytophtora ramorum, the pathogen responsible for the disease known as sudden oak death. To assess the survival rate of the pathogen in compost, we introduced zoospores — a type of infectious propagule — into six composts of varying provenance and maturity. The compost samples represented three production facilities, two production techniques (turned windrow and forced air static pile) and two levels of maturity (fresh, defined as aged for less than 1 week; and mature, aged for more than 4 weeks). Positive re-isolations — indicating survival of the pathogen — were obtained from all composts. The re-isolation rate from the compost from one of the three production facilities was greater than that obtained from an inert substrate (filter paper) inoculated with the pathogen (P < 0.01), while re-isolation rates from the other two sources were statistically indistinguishable from those obtained from the inert substrate (P < 0.01). There was no significant difference in re-isolation rate between composts produced by the turned windrow method and composts produced by the forced air static pile technique. Re-isolation rates were greater from mature composts than from fresh composts (P < 0.01). The results show that P. ramorum may be present and infectious if introduced into finished compost, and that variations in compost characteristics appear to influence survival rates.
Full text
Phytophthora ramorum, the causal agent of the disease commonly referred to as sudden oak death (Rizzo et al. 2002), has killed millions of trees on the north coast of California. (Frankel and Palmieri 2014; Meentemeyer et al. 2011). An introduced pathogen both in North America and Europe (Goss et al. 2009), it was discovered in California in 1995. P. ramorum often forms lethal bark lesions on oaks (Quercus spp.) and the related tanoak (Notholithocarpus densiflorus), but it spreads by spores formed on foliar lesions on scores of other plant species, including common landscape plants (Rizzo and Garbelotto 2003). New foliar hosts have been discovered annually since 2002 (USDA-APHIS 2013), and the symptoms can vary substantially from host to host (Garbelotto 2003; Hüberli et al. 2003; Hüberli et al. 2004; Murphy and Rizzo 2003). Furthermore, the disease keeps spreading to new locations through limited-distance natural dispersal, infected nursery stock and perhaps through other yet unknown means (Croucher et al. 2013; Garbelotto et al. 2003; Orlikowski and Szkuta 2002; Werres et al. 2001). To help prevent the spread of the pathogen to new localities, movement of infected plant material is highly regulated (Paswater 2003).
UC ANR researchers tested composts made from municipal green wastes to determine whether the sudden oak death pathogen could survive in finished compost.
With the host species list and associated symptoms growing at this rate, even conscientious landscape contractors may not be able to keep pace and identify those plants likely to be infected. Debris from infected plants is almost certainly taken to local composting facilities, which are subject to restrictions on shipping product out of the quarantine area if found to be not pathogen-free (Paswater 2003). Leaves of foliar hosts can be extremely infectious (Davidson et al. 2002), even for extended periods of time. For instance, P. ramorum can remain viable on detached leaves of bay laurel (Umbellularia californica) for several weeks (Harnik et al. 2004). Survival of P. ramorum in dead and down logs and firewood (Shelly et al. 2005) can also last several weeks.
The composting process can eradicate even the toughest resting propagules commonly produced by P. ramorum if the process is conducted according to U.S. Environmental Protection Agency (EPA) PFRP guidelines (Swain et al. 2006).
In addition, finished compost has a well-established history of suppressing a variety of plant pathogens when incorporated into potting mixes or planted into soil (Hoitink and Fahy 1986), though these suppressive qualities have primarily been demonstrated once the compost has been incorporated into soil or container media (Bollen 1985; Gorodecki and Hadar 1990; Hoitink and Boehm 1999), and may not be an inherent property of the finished compost itself (Hardy and Sivasithamparam 1991). The survival of P. ramorum in finished compost, however, had not previously been evaluated.
It stands to reason that P. ramorum would be able survive in finished compost if resting propagules are introduced directly into it, as such propagules have been isolated and germinated from inhospitable substrates including tires and sneaker soles (Davidson et al. 2005).
We use the term resting propagules to refer to spores such as chlamydospores and oospores that have thick cell walls resistant to desiccation, microbial degradation and temperature extremes, as might be found in compost piles. Other sources have referred to these structures as survival propagules (Erwin and Ribeiro 1996), or resting spores (Judelson and Blanco 2005). Resting propagules are comparatively large and heavy, and as such they don't disperse as well as other spore types such as sporangia or zoospores (Judelson and Blanco 2005). We distinguish resting propagules from dispersal propagules, which are more delicate spore types such as sporangia and zoospores. Sporangia are light and passively carried by wind and rain, while zoospores actively swim in water films and hunt for suitable hosts to infect.
Our research addressed the question of whether P. ramorum may have a high survival rate in finished compost if reached by dispersal propagules that may be transported by wind or water from fresh green waste or infectious plants within or near composting facilities. In other words, we sought to determine whether finished compost allows for survival of P. ramorum, assuming: (a) inclusion of resting propagules such as chlamydospores has been avoided and (b) dispersal propagules have reached it. In addition, we investigated whether compost can become infectious — that is, whether re-isolation rates of dispersal propagules from finished composts can be higher than those from any similarly treated inert substrate.
Materials and methods
In order to maximize the differentiation between survival — a process mediated primarily by the survival and germination of resting propagules — and an infectious phase in which secondary sporulation and creation of dispersal propagules may occur in the absence of resting propagules, we developed a water bath inoculation system that would allow for production of dispersal propagules — in this case, zoospores — without significant introduction of resting propagules. Accordingly, the timeline of this experiment was designed to study zoospore driven colonization, as this is the process that is the primary driver behind the “natural” infection process of P. ramorum (Garbelotto and Hayden 2012).
We used finished composts of varying provenances and curing times, produced both by “turned windrow” and “forced air static pile” techniques. The term “finished” here refers to compost that has completed its thermophilic phase. After the thermophilic phase is completed, most commercial composts are cured for a time ranging from a few days to several months, depending upon the production system used and the characteristics of the desired end product (Wu et al. 2000). During the curing phase, phytotoxic chemicals are degraded and metabolic rates within the compost are given time to stabilize. This process is important to the production of most commercially produced composts (Wu et al. 2000), and composting facilities typically have large piles of curing compost on site. Were P. ramorum to be introduced into these piles and to survive, it could be transported to new uninfected locations when the compost is sold.
Inoculation methods and substrates
Using zoospores.
Three 1.5-cm diameter disks each of P. ramorum isolate Pr52 (CBS110537; ATCC MYA-2436) and Pr102 (ATCC MYA-2949) grown on V-8 agar (Erwin and Ribeiro 1996) were placed into each 90-mm petri dish and flooded with enough deionized water to bring the level just below the surface of the agar disks. The dishes were incubated in the dark at 16°C for 3 days, then chilled to 4°C for half an hour to induce sporulation, and then incubated for 1 hour at room temperature before quantifying zoospores with a hemacytometer (Hausser Scientific, Horsham, PA). Zoospore concentrations were diluted to 5 × 104 zoospores/ml, and 15 ml of the inoculation solution was poured into inoculation cages (see below).
Using colony plugs.
Three 1.5-cm diameter disks each of P. ramorum isolate Pr52 (CBS110537; ATCC MYA-2436) and Pr102 (ATCC MYA-2949) grown on V-8 agar (Erwin and Ribeiro 1996) were used for inoculation. Such plugs contained hyphae (filaments that make up the body of P. ramorum), sporangia and chlamydospores, and were placed directly into the inoculation cages (see below).
Compost.
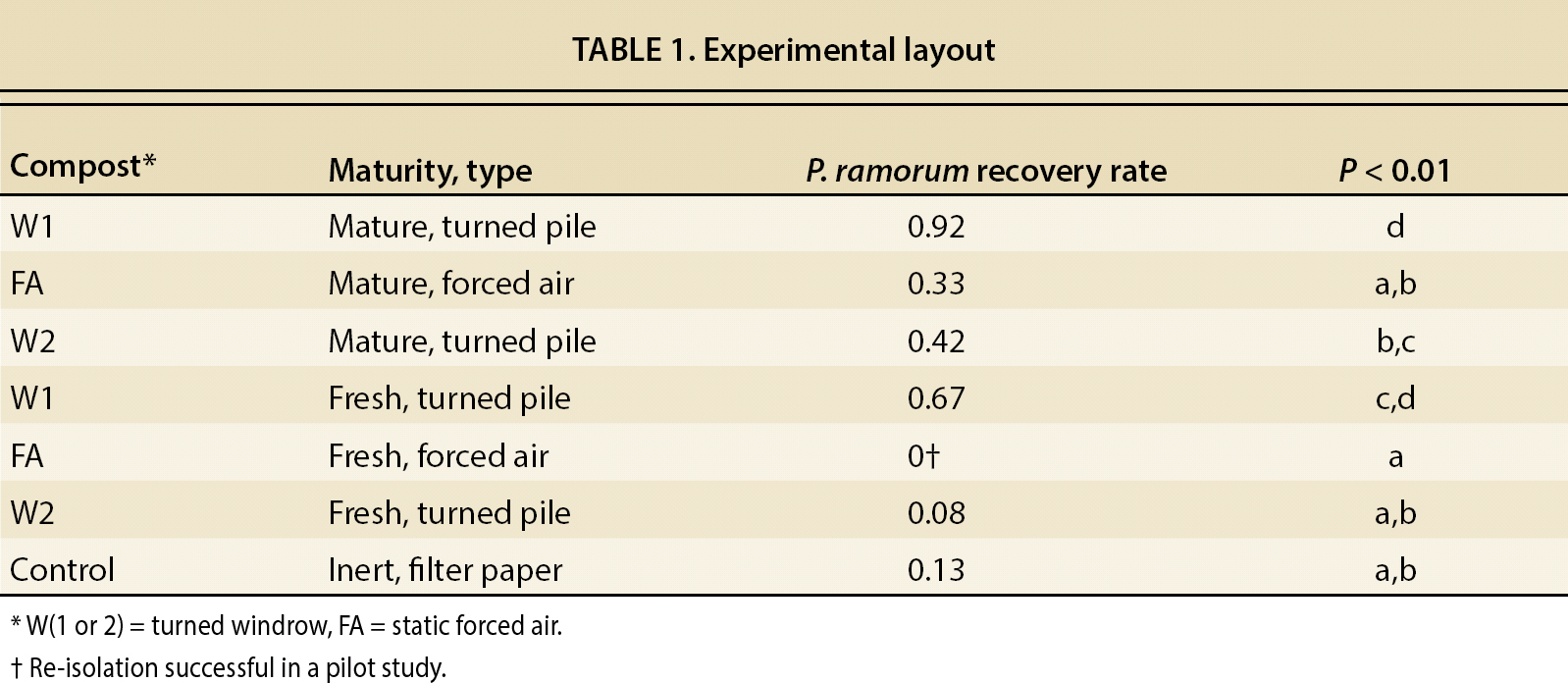
The composts used in these experiments (table 1) were sourced from three different commercial suppliers. Two of the suppliers (which produced composts W1 and W2) used turned windrow method, while the third used forced air static pile composting (compost FA). All three composts were made from municipal green wastes sourced from the northern San Francisco Bay region. Two categories of compost were used: (1) “Fresh” composts, which had just finished their thermophilic phase, and had been curing for less than one week, and (2) “mature” composts, which had been curing for 4 weeks or more. Composts came from commercial facilities where temperatures and time of composting follow EPA guidelines (EPA 2003). Each compost included a control treatment and was tested by pear baiting to ensure it was free of any phytophthora prior to being used in the experiment.
Filter papers.
All experiments were replicated using filter paper as an alternative media to compost, in order to separate any compost-specific results from results that could be obtained from any inert media.
Water baths.
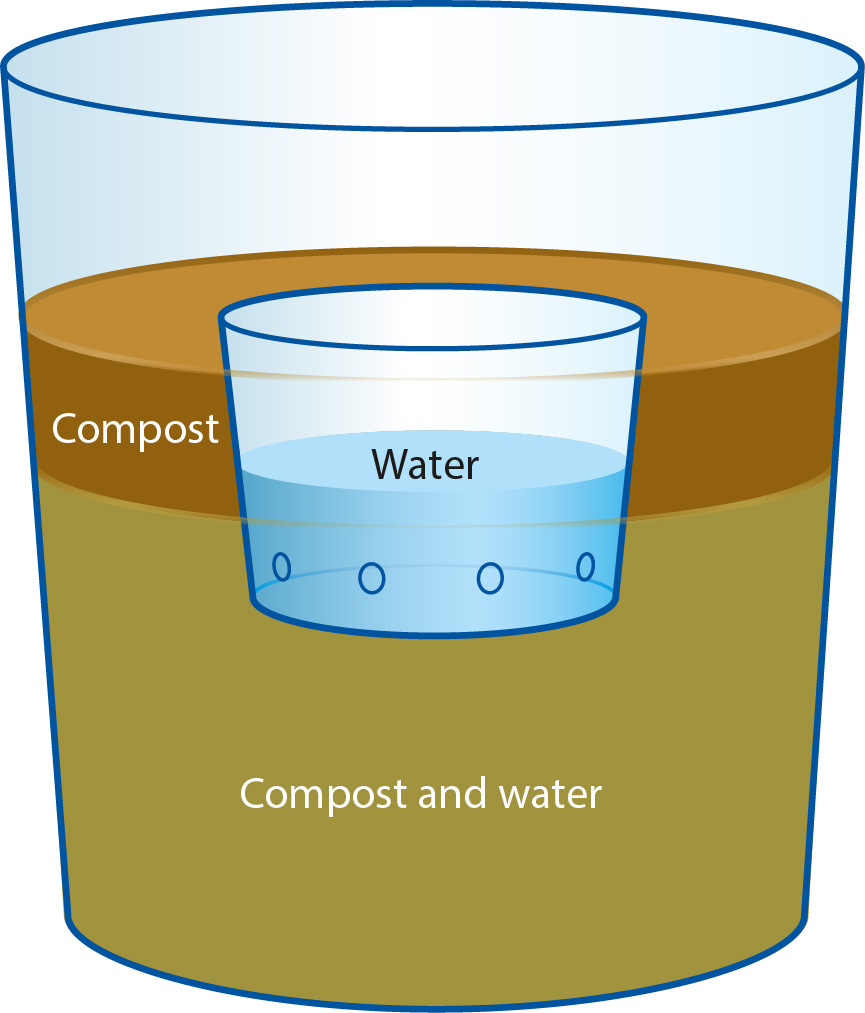
Each compost or filter paper was placed into a 1-quart ice cream container, and partially flooded with deionized water. A cage, made from a small perforated plastic cup, was then placed into the compost-water mixture, and the inoculum was introduced to the cage (fig. 1). For the hyphal series, whole inoculum grown on agar plugs was placed into the cages. For the zoospore series, approximately 15 ml of zoospores were introduced to each cage. The compost was then flooded the rest of the way, allowing the water to flow into the cup from the outside. The resulting assemblages were then stored in the dark at 12°C for 4 days, after which time the cages were removed with their contents. Finally, the bottoms of the ice cream containers were perforated, allowing the water to drain off. The compost and filter papers were then allowed to dry until each substrate was moist. Half of each filter paper was cut up and plated as outlined in Direct testing, below. The remaining half filter papers, and all compost samples, were then transferred into their own 1-gallon plastic bag and pear baited. Negative controls were run where no inoculum was added to each cup. For positive controls, washed, green, unripe D'Anjou pears were added to the ice cream containers in place of compost or filter paper, and the perforated plastic cages were affixed to the sides of the containers with tape. This process was simultaneously replicated four times for each compost origin and age. The entire series was simultaneously replicated twice more, resulting in 12 containers for each compost type, split into three replicates of four.
Fig. 1. One-quart ice cream container water bath diagram. For positive controls, pears were placed into containers containing no compost. For negative controls (inert media) filter papers were placed into the bottoms of the containers instead of compost. Perforated cage for zoospores or culture disks is shown sunken in compost and water.
Pathogen re-isolation and analyses
Pear baiting.
A single green, washed, organically grown unripe D'Anjou pear was placed into each 1-gallon plastic bag or 1-quart ice cream container, and enough water was added to the container to cover most of the pear, and/or achieve an approximate water to compost ratio of 4:1 (Tsao 1983). The pears were incubated in the water for 4 days and then placed to dry on paper towels for an additional 4 to 5 days, all at 12°C. The resulting pear lesions, if found, were then plated onto PARP as outlined below in Direct testing.
Direct testing.
Viability of the pathogen was determined by counting how many filter paper or pear skin sample fragments (about 1 to 4 mm2 in size) formed colonies when plated on P10ARP (PARP) selective media (Erwin and Ribeiro 1996, modified to 25 µg/ml PCNB). One sample was plated directly onto PARP selective media from the infection margin of each pear lesion; approximately 50 sample fragments from one half of each filter paper were plated onto PARP as well. PARP plates were incubated in the dark at 20°C and scored as either positive or negative at 2 weeks from the time of inoculation. As a positive control, one plate from every batch of PARP medium was infected with Pr52 and Pr102. Any batch of PARP that failed to support the growth of P. ramorum was discarded.
Statistics.
All formal analysis was done on a pairwise basis using nonparametric Fisher's exact test.
Results
A contingency analysis was performed comparing isolation success of composts inoculated using zoospores and those inoculated using hyphal plugs bearing sporangia. Results show no difference between the two inoculation methods (N = 118, DF = 1, –LogLike = 0.21870008, R2 = 0.0027; two-tailed Fisher's exact test P = 0.5726) and hence data from the two were pooled together.
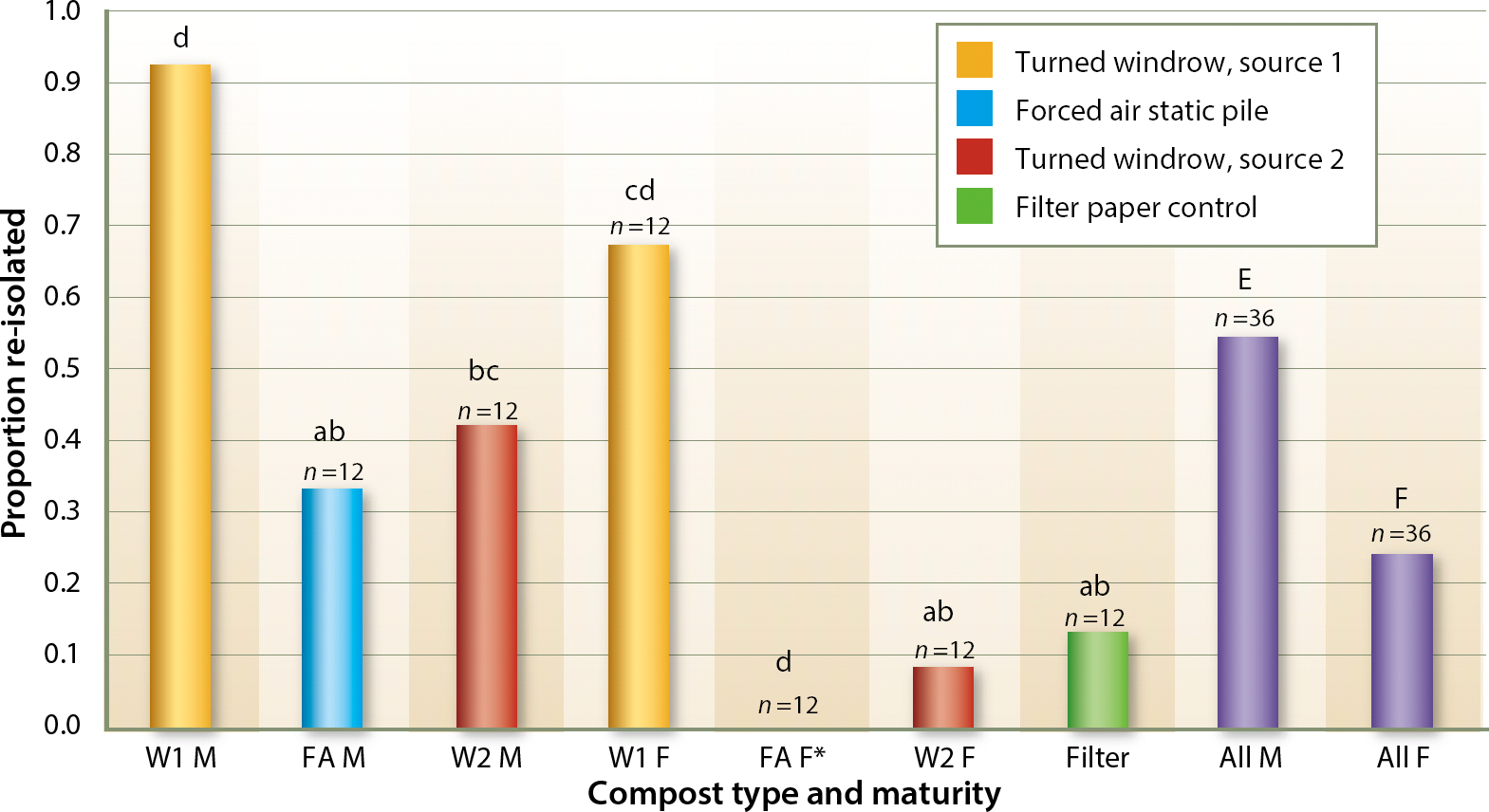
It was possible to recover P. ramorum from all composts except for fresh compost from site FA (table 1). However, low levels of P. ramorum were recovered from fresh site FA compost in a pilot study previously completed (data not shown). Recovery from “mature” composts that had cured for 4 weeks or more (M) was higher than that from “fresh” composts that had cured for less than 1 week (F) (P < 0.01).
Substrates were clumped in two groups (labeled “a” and “d” in fig. 2 and table 1) (P < 0.01) based on recovery rates: the highest recovery of P. ramorum was obtained from both mature and fresh turned pile composts from site W1, while the lowest recovery rates were obtained from the inert substrate and from all other composts except the mature turned windrow pile W2. The mature turned pile compost W2 had an intermediate recovery rate overlapping the two groups. It is also interesting to note that the recovery rate of the fresh forced air compost FA was lower than that of the inert substrate (fig. 2).
Fig. 2. Percentage comparison of re-isolation success. Column suffix M denotes mature compost (inoculated after it had aged 4 weeks or more), while column suffix F denotes fresh composts aged less than 1 week. Columns labeled “all” combine either mature or fresh compost from all sources, and therefore show average values across all facilities and composting techniques used. Letters denote significance grouping at the (P < 0.01) level of confidence. Lowercase letters can be compared to other lowercase letters, while uppercase letters should only be compared to other uppercase letters, as significance was not cross checked across case groupings. Any column not containing a given letter is statistically different at P < 0.01 from any column that does, except as stated above. For example, compost pile W2 F (a, b) is similar to any piles marked a or b, but it is statistically distinct from piles marked c or d. * Re-isolation successful in a pilot study.
Discussion
P. ramorum could be recovered from every compost substrate tested, so it is clear that at least some, and possibly all, finished composts allow for survival, even when the number of dispersal and resting propagules is minimized. The recovery rates for FA and W2 composts were statistically indistinguishable from filter paper, which suggests that these composts are not any better substrates for P. ramorum survival than any other material. Interestingly, the recovery rate from fresh forced air compost FA was lower than that from the inert substrate, suggesting this composting technique generates a substrate unfavorable to survival of P. ramorum zoospores. It appears that this peculiarity may be lost as the forced air compost matures, suggesting that the low survival rate of the pathogen in fresher compost may be due to a transient presence of inhibiting chemicals or competing thermophilic organisms, or both.
Recovery rates obtained from site W1 were significantly higher than those from inert media and from the other two composts. The higher rates most likely simply reflect a higher survival rate, though another possibility is a higher germination rate after encystment (Erwin and Ribeiro 1996), and while it's unlikely, we cannot exclude the possibility of colonization.
Overall, fresh compost was less favorable to P. ramorum recovery than mature compost, suggesting that well-cured compost may represent a greater risk for spreading P. ramorum if it is infected. The lower suppressive action of older compost is expected, due to changes in microbial communities and in particular due to a lower representation of highly suppressive thermophilic fungi in older composts (Goyal et al. 2005).
Although we did not test for how long each substrate remained infectious, our main goal here was to determine whether P. ramorum, a federally regulated pathogen (USDA 2007), may be present and infectious in a commercially available product, were it to be infested. Our results show that it can, and that there are initial differences among substrates. How long each substrate will remain infectious is relatively less important when dealing with a regulated organism.
At present we cannot explain the source variation found between composting facility W1 and facilities FA and W2. A large number of factors may be involved, including the base materials going into the compost (Hoitink and Boehm 1999), the moisture, carbon availability and fungal diversity of the pile (Soares et al. 1995), and the frequency and efficiency of turning operations (Churchill et al. 1995). The most apparent difference between the two windrow composting facilities is that facility W2 has specially designed windrow turning equipment (Scarab compost turner), while facility W1 uses front-end loaders for turning. Furthermore, compost from site W1 appeared to be composed of materials that were ground more coarsely prior to composting than the other composts, so it appears that finer composts may be more suppressive than coarse ones.
Conclusions
Our study was designed as a proof of concept and the conclusions we can draw from the results are that P. ramorum can survive if inoculated using a high concentration of inoculum in all finished compost, that older finished composts are less suppressive than fresh finished composts, and that it may be able to grow in some composts. However, our study did not address which traits may make a compost suitable for growth of the pathogen: further research including precise characterization of composts using the U.S. Composting Council Test Method for the Examination of Composting and Compost (TMECC) standards ( compostingcouncil.org/tmecc/ ) is needed to determine which types of composts are most amenable to survival and possibly growth of this serious plant pathogen.
It should be noted that our experiments involved the use of large amounts of inoculum. Under real-world conditions a comparable situation might only occur when large amounts of fresh infected plant material is shipped to the composting facility in cool, rainy conditions, or if compost rows were to be under or near highly infected infectious hosts such as Rhododendron spp., California bay laurels or tanoaks.
These findings suggest that in composting facilities that may be shipping material out of the immediate area, measures should be taken to ensure that finished compost is not contaminated by infected green waste. Best management practices for composting facilities should minimize the potential for infected surface water or wind-blown rain from fresh materials to contaminate mature compost. Additionally, known plant hosts for P. ramorum should not be located within the immediate vicinity of the composting facility. We encourage monitoring of infectious hosts near composting facilities within the zone of infestation (see sodmap.org ), and their removal, if possible, when these plants may be within the facility itself. These measures are essential to ensure the final product does not include any infectious material.