All Issues
New pests and diseases: Sudden oak death syndrome fells 3 oak species
Publication Information
California Agriculture 55(1):9-19. https://doi.org/10.3733/ca.v055n01p9
Published January 01, 2001
PDF | Citation | Permissions
Abstract
“Sudden oak death” refers to a complex set of symptoms that has already culminated in the death of tens of thousands of California oak trees. Now confirmed in seven coastal counties, SOD attacks California tanoak, coast live oak and California black oak. Although several fungal species and the western oak bark beetle and ambrosia beetles have been associated with the syndrome, we now have solid evidence that a newly discovered Phytophthora species is the primary causal agent. This Phytophthora species was recently isolated in rhododendron as well; it may be the same species that was isolated, but not described, on rhododendrons in Germany in 1993. The discovery of SOD on rhododendron has serious implications. The disease may well be present at the ecosystem scale, and its appearance on an ornamental plant suggests the possibility of wider dissemination. A team of UC scientists has developed an integrated approach to managing this disease, including practices to enhance tree health. Early disease detection and targeted chemical treatment may also hold some promise for disease management. In addition, we have developed a molecular probe that will enable rapid identification of SOD from any infected part of the plant. Ultimately, the fate of the oak species affected by SOD will be determined by the levels of disease resistance present in natural populations of these trees.
Full text
Sudden oak death syndrome killed about 350 coast live oak trees in this area near Mill Valley in Marin County. This photo was taken in March 2000.
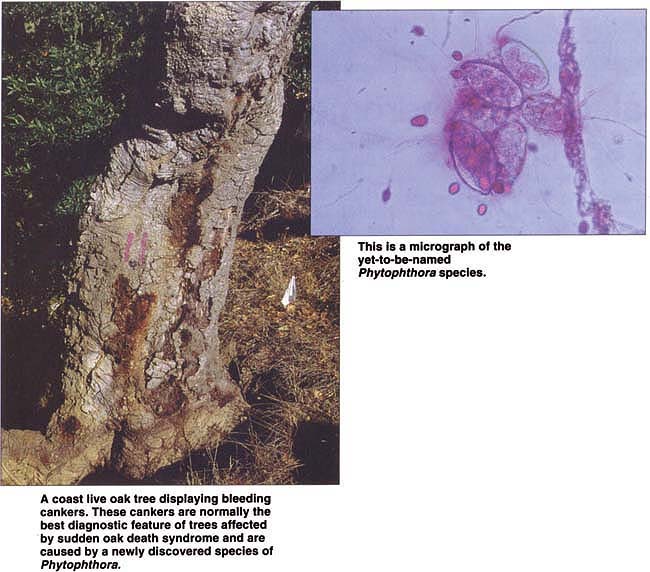
A coast live oak tree displaying bleeding cankers. These cankers are normally the best diagnostic feature of trees affected by sudden oak death syndrome and are caused by a newly discovered species of Phytophthora.
Since 1995, mortality of at least three native California oak species has reached epidemic levels (McPherson et al. 2000). The seemingly rapid decline of these trees is triggered by a new species of Phytophthora (a funguslike organism). Named sudden oak death (SOD), or oak mortality syndrome, this disease has never before been observed in California. SOD has received substantial attention by the media and the public because oaks not only represent a major component of many California forest ecosystems, they are also important both in urban landscapes and at the urban/rural interface. Tanoak, coast live oak and black oak trees are distributed along 1,500 miles of the California and Oregon coast. Researchers, land managers and policy-makers are using adaptive management to direct their efforts, but are faced with the challenge of formulating control guidelines based on a limited and still evolving understanding of the disease.
This article summarizes the current knowledge about sudden oak death and refers to some of the ongoing experiments designed to provide insights into its possible causes. Finally, we address potential options for control. Because this work is in progress, most of this information should be treated as preliminary. Often we rely on references to similar but not identical problems in other systems. The information presented here has been condensed from the work of many researchers actively involved in the study of SOD. Among them are Susan Frankel, Thomas Gordon, Kent Julin, Maggi Kelly, Steve Koike, Brice McPherson, Nicole Palkowsky, Garey Slaughter, Andrew Storer, Ted Swiecki, Steve Tjosvold, Ellie Rilla, Rick Standiford and David Wood.
What is sudden oak death?
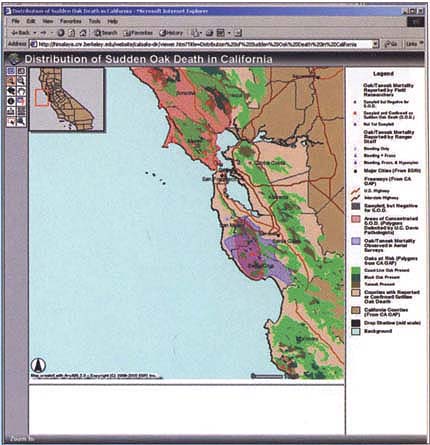
Sudden oak death (Svihra 1999) is known to affect three tree species: California tanoak (Lithocarpus densiflora), coast live oak (Quercus agrifolia) and California black oak (Q. kelloggii). We have also isolated the pathogen from rhododendrons. Although SOD has been reported only in central and northern coastal California (fig. 1), the natural range of tanoaks and black oaks extends well into the interior ranges of California and into Oregon. Coast live oak is found throughout Southern California and into Baja California, Mexico. In addition, the presence of SOD on a commercial ornamental plant (rhododendron) raises the possiblity that it may already have been disseminated more widely than we know. Its presence in ornamental plants could be temporarily masked by the use of fungicides.
We still do not understand patterns and mechanisms of dispersal of this new disease. Therefore it is not known whether SOD will expand throughout the range of its hosts, or whether its distribution will be limited by factors such as climate, presence of competing organisms and predators, or absence of potential vectors. The disease is currently patchy in its regional distribution, but can affect 40% to 80% of trees in any given stand. Such high levels of oak mortality are unprecedented in California, and suggest the emergence of a new disease.
Trees affected by SOD display a range of symptoms depending on stage of infection, tree species and the time of year. Wilting of apical shoots is a common initial symptom of infected tanoaks. Sparse pale-green foliage is observed in coast live oaks in an advanced stage of the syndrome. Mature trees of all three host species affected by SOD display bleeding and sunken cankers on the bole, or trunk. These cankers are more frequent on the lower part of the trunk, but they can be found up to 30 feet (10 meters) from the base of the tree. Cankers seep a dark brown or amber-colored sap. Such seeping is the most dependable symptom of a tree affected by SOD. Intensity of seeping varies among different trees and may fluctuate on the same tree, perhaps depending on the time of year or stage of canker development. For instance, seeping tends to decrease during dry periods and in older cankers. In smaller tanoaks, no seeping may be associated with the cankers, which appear water-soaked and may be scattered on stems.
S.O.D. Phytophthora basics
Phytophthora species produce both sexually and asexually, and the gametes and clones that result can survive as spores for extended periods, even in adverse environments.
In the new SOD Phytophthora, only asexual structures have been observed to date. This may be favorable. Pathogens without the ability to recombine their genetic information through mating can be aggressive but are less capable of rapidly adapting to new environments or hosts.
Scientists have observed that this species produces two types of infectious structures called sporangia and chlamydospores, both capable of being detached from the main body of the pathogen. It follows that spread may occur through air, water, soil or animals. However, actual methods of transmission have not been confirmed in the field.
The following definitions are derived from Ainsworth's Dictionary of the Fungi, with additions by the authors relevant to SOD.
Chlamydospore: A thick-walled asexual spore made by the rounding up of one or more cells. It can survive for up to several months or years in unfavorable conditions, it is known to be responsible for long- and medium-range movement in contaminated soil and water of many pathogens including the Port Orford cedar Phytophthora.
Oosphere: The female gamete, similar to the “egg.”
Oospore: The resting spore from a fertilized oosphere. The structure is the result of mating and genetic recombination.
Sporangium: An organ producing asexual spores. It is not the result of mating; no genetic recombination occurs. In this case it is lemon-shaped and the spores have two flagella (whiplike extensions for movement). The sporangium can detach itself from the body of the pathogen, germinate directly and infect the host without the need to open up and release the zoospores.
Zoospores: Asexual spores with flagella. (In virtually all Phytophthora species, flagella enable spores to swim; they can move towards susceptible infection sites by following chemical signals from the plant.) If they do not find a susceptible host they can encyst for variable periods of time and wait for more favorable conditions. They do not survive for more than a few days, however.
Fig 1. Counties infected with the new species of Phytophthora that causes sudden oak death syndrome (SOD).
In later stages of SOD, coal-black dome-shaped fruiting bodies of the fungus Hypoxylon thuorsianum invariably develop. At first the black fungal domes appear near and above the canker areas and then over large portions of the bole. Beetles (Coleoptera: Scolytidae) are also consistently found on trees in later stages of SOD. The western oak bark beetle (Pseudopityophthorus pubipennis) and ambrosia beetle (Monarthrum spp.) feed respectively on the bark (phloem) and sapwood of host trees. Their feeding activity results in barely visible tunnels on the tree bark and in the production of finely ground frass (chewed phloem or sapwood) that accumulates, often in large amounts, on the tree bark. We believe that in spite of the strong association between Hypoxylon and/or beetles and tree mortality, these organisms should be considered as secondary. Such organisms are present on declining oaks worldwide, no matter what the cause of the decline.
We have isolated an oomycete fungus belonging to the genus Phytophthora from many trees with SOD symptoms. Many oomycetes are aggressive plant pathogens, including the pathogen responsible for the infamous Irish potato blight of the 19th century. We succeeded in isolating Phytophthora from SOD-related seeping cankers at the root crown to over 10 feet (3 meters) above the ground. Seeping, a likely host defense response, may provide the fungus with a potential route of dispersal for its infectious propagules. These propagules, called sporangia, have been found in the sap oozing from cankers during wet and cool periods. From there, the sporangia may either be splashed onto other trees or possibly transported by animals. Infection on a new host begins in the phloem, progresses to the cambium and eventually reaches the xylem. The phloem is often a bright red with numerous thin black lines that delimit the canker. Infection and discoloration are always more extensive in phloem tissues than in xylem. Black discoloration can extend up to 3/4 inch (2 cm) into the xylem below the bleeding canker. Discoloration in the phloem and xylem may extend up to 20 inches (50 cm) in all directions from the point of bleeding. Phytophthora is typically isolated from the margins of these discolored areas, although it can often be isolated from older portions of the cankers. Cankers on smaller tanoaks are not as distinct, and zone lines are not always apparent in either phloem or xylem.
Elevated oak mortality caused by other species in the genus Phytophthora has been reported from other regions of the world (Brasier et al. 1993, Jung et al. 1999, Tainter et al. 2000). For decades, a few Phytophthora species (P. cinnamomi, P. citricola and P. cactorum) have been known to be present on oaks in California. However, the newly isolated Phytophthora does not match the morphology of any of the known described species (Erwin and Ribeiro 1996), and is still unnamed. In this paper, we refer to this new species as SOD Phytophthora. DNA analysis also indicates that this is a new undescribed species, whose only close relative is another Phytophthora species (P. lateralis), which is present only in California, Oregon and Washington. This related pathogen, considered by many to be an exotic species, is extremely virulent on Port Orford cedar (Chamaecyparis lawsoniana) in the Pacific Northwest. Although the two species of oomycetes are morphologically different and attack different tree species, they share the same requirement for cool climates, they do not tolerate temperatures over 95°F (35°C). Both species also produce similar chlamydospores, thick-walled propagules that ensure survival of the pathogen in harsher climatic conditions or unfavorable environments.
Diagnosis of SOD: case study of a scientific process
Identifying the character and cause of sudden oak death (a previously undescribed Phytophthora species) was a tortuous, 5-year process.
It began in April 1995 when homeowners asked the UC Cooperative Extension office in Marin County to investigate the dieback of more than a dozen tanoaks bordering a creek. The root crown area beneath the bark of the largest tanoak showed mats and strands of “shoe-strings” that are typical of the oak root fungus. Armillaria mellea. These oaks are usually tolerant of this pathogen and can withstand some drought. However, it was thought that prolonged drought from 1990 to 1992, followed by very wet years in 1993 and 1994, might have reduced the vigor of tanoaks, allowing A. mellea to infect and kill them.
In June 1996, a reexamination of the dying tanoaks revealed a shocking number of dying and dead trees, not only along a creek but also on the slope and along the crest of a hill. Symptoms could no longer be attributed to A. mellea. The current year foliage of other trees had turned yellow-green and new shoots drooped, typical of trees with root disease. Trees exhibited bleeding cankers and heavy infestations of ambrosia beetles, signalled by piles of fine white-to-fan sawdust on the lower trunks. Dead trees also had charcoal-black spherical fungal fruiting bodies on the bark surface.
With the cooperation of arborists, horticulturists and pathologists, intensive sampling of affected trees was conducted in June, August and October 1996. Samples of discolored tissues and wilted twigs were sent to UC and state laboratories, but no cause was identified. Scientists proposed that the mysterious dieback could be caused by the devastating chestnut blight Cryphonectria parasitica, or perhaps oak wilt caused by Ceratocystis fagacearum. Again, laboratory tests followed, but neither fungus was detected.
Coast live oak dieback
In May 1997, the first coast live oaks, Quercus agrifolia, began to die in gardens of Marin County. The dead trees were also experiencing stressful environmental conditions. The coast live oak symptoms were strikingly similar to those of dying tanoaks, especially the heavy ambrosia beetle infestations, and the growth of a charcoal-colored hemispheric fungus on the bark. However, scientists also noted that some young tanoaks died in the vicinity of mature, dead tanoaks without noticeable bleeding or ambrosia beetle attacks.
In June 1997, we examined two Mill Valley tanoaks that were 6 inches in diameter, adjacent to each other, and the targets of initial ambrosia beetle attacks. The first tree died during the winter season; the second had yellow foliage with typical wilted shoots. Both trees were uprooted with a back-hoe. Samples were collected of necrotic or discolored roots, inner bark, and cambium tissues under the patches of ooze, and branches and shoots that showed discoloration, streaks or staining. Three fungi and one bacterium were identified in laboratory tests, but none of these organisms was considered by plant pathologists to be the primary agent of tree mortality.
About the same time, the California Department of Food and Agriculture laboratory identified three beetles from infested trunk sections: two ambrosia beetles and one bark beetle.
Characterization and control
During the fall and winter of 1997 many more dead trees appeared. As no pathogen could be causally implicated in the dieback, which continued to expand, UC Cooperative Extension in Marin County formulated management recommendations in spring 1998.
It seemed probable that drought followed by excessive rains had caused stress in the affected trees. As a result, bark beetle and ambrosia beetle populations built up in dying tanoaks to an epidemic stage. The new beetle generations not only attacked tanoaks but also shifted their attacks to coast live oaks. Through meetings and workshops with arborists, UCCE's Marin County office implemented a program of prophylactic insecticidal sprays combined with tree health care (irrigation, cleaning of root crown areas, pruning, fertilization, etc.).
In June 1998, soon after the treatment program began, there was a substantial increase in dead coast live oaks from Mill Valley to Novato, and two black oaks, Quercus kellogii, died in Novato. The symptoms of the black oaks were almost identical to those of dying tanoaks and coast live oaks. For the first time, the word “epidemic” was used widely and the CE advisor informed the media. However, scientists did not agree on the definition or diagnosis for the problem because new inconsistencies continued to emerge. For example, bark beetles and ambrosia beetles were considered the primary cause of the death in coast live oaks and black oaks because these trees were predisposed by environmental stress. However, the death of very young tanoaks that lacked ooze on the trunk and were not attacked by beetles seemed to contradict insects as the primary cause. It appeared that an unknown pathogen was traveling from mature dead trees to kill these young trees.
Rising tree deaths
In winter 1999 the number of dying trees continued to rise into the tens of thousands. A San Rafael resident counted 316 dead trees on Mountain View Avenue. At the same time, an opinion circulated that the oak die-back was a temporary phenomenon (a “natural cycle”) related to climatic extremes. In June 1999, the California Forest Pest Council met to assess the massive death of oaks. Cooperating plant pathologists uprooted a symptomatic tanoak tree and collected samples. No plant pathogens were recovered. Several “Pest Alerts” were published (Svihra 1999, 1999a, 1999b, 2000). One described the syndrome as “sudden oak death,” and the term stuck.
Scientific breakthrough
In June 2000, David Rizzo, UC Assistant Professor of Plant Pathology, sampled infected coast live oaks in the China Camp State Park near San Rafael and returned to his laboratory on the same day to make isolations from the tissue samples. A fungus of the Phytophthora genus was isolated from the tissue and appeared to be the underlying cause of SOD (McPherson et al. 2000). This rapid processing of tissue samples was essential to recovery of the pathogen. Previous samples that were mailed or even hand-delivered had not revealed the cause.
Currently, there are no measures available that will alter the underlying disease. The potential for fungicides, other management practices and wood disposal to mitigate the impact of the disease is currently under investigation. In vitro studies have demonstrated that some fungicides may slow down or prevent the underlying disease development process. However, the effectiveness of these and other control methods have not yet been confirmed in the field, nor have potential problems of resistance been assessed.
References
Svihra P.. Sudden Death of Tanoak, Lithocarpus densiflorus. UC Cooperative Extension 1999. Junep.2. Pest Alert #1
Svihra P. Western Oak Bark Beetles and Ambrosia Beetles, Killers of Live Oaks. UC Cooperative Extension 1999a. Junep.2. Pest Alert #3
Svihra P. Western Oak Bark Beetles and Ambrosia Beetles, Killers of Live Oaks. UC Cooperative Extension 1999b. Octoberpp.1-4. Pest Alert #3A
Svihra P. Protection of Live Oaks Against Attacks by Oak Bark Beetles and Ambrosia Beetles. UC Cooperative Extension. 2000. January1-4. Pest Alert 3B
McPherson BA, Wood DL, Storer AJ, et al. Oak Mortality Syndrome: Sudden Death of Oaks and Tanoaks. Tree Notes. 2000. (August)266-
The role played by chlamydospores and most of the biology of the SOD Phytophthora is still unknown. Phytophthora lateralis and many other Phytophthora species are dispersed through the movement of soil and water contaminated by chlamydospores. This mode of dispersal needs to be verified for the SOD species. Whether and for how long the pathogen can survive in dead wood is also still undetermined. Although our observations indicate that the pathogen can infect trees by penetrating directly through the unbroken bark, the importance of wounds and of potential vectors needs to be investigated.
One feature that appears to differentiate the SOD Phytophthora from other forest Phytophthora species is the presence of an aerial component. This component, inferred from the presence of aerial cankers, can be determined by the cauducous nature of the sporangia. This feature may allow these infectious propagules to detach from the fungal colony and become airborne. A significant aerial component in the disease cycle may also explain the lack of a strict association between patterns of disease spread and the presence of water. Such association is normally observed for nonaerial Phytophthoras.
Understanding whether the SOD pathogen may be (a) introduced; (b) a new taxonomic entity obtained by hybridization between P. lateralis and another Phytophthora species; or (c) a native pathogen may help in predicting the final impact that SOD is going to have on oak ecosystems. Non-native or new diseases often find plant hosts ill-equipped to defend themselves, and the result can be host decimation. Native diseases may cause epidemics due to increased host predisposition, bu they rarely wipe out entire host species.
We also do not know whether or not this pathogen completes its sexual cycle. In general, pathogens that are capable of completing the sexual cycle are more successful both in adapting to new environments and in developing resistance to compounds used for chemical treatments.
Probable primary causal agent
Although SOD may ultimately be the result of a complex scenario in which several pathogens, pests and abiotic factors interact with one another, the new Phytophthora seems to be found on all trees affected by SOD. This was evidenced by direct isolations of the pathogen from the hosts or by the presence of typical sunken cankers and seeping. This strong association suggests that this organism is the primary causal agent of the syndrome. Inoculations of seedlings, saplings and adult trees have been performed to confirm its pathogenicity. Preliminary results from all three experiments indicate that this oomcycete is capable of causing disease symptoms on inoculated hosts comparable to those in naturally infected plants.
Although there is also a striking correlation between SOD and both Hypoxylon and beetle attacks, we believe that these and other organisms may play a secondary role. Fungi in the genus Hypoxylon are normally endophytically present and inactive in the host, until mechanical damage, insects or diseases create the right environmental conditions for their growth. Twig or stem cankers caused by fungi other than Phytophthora (for example, Botryosphaeria spp.) are also often abundant in areas affected by SOD. These are considered as secondary or opportunistic organisms that thrive on trees weakened by other factors. The correlation between insect attacks and dead trees is striking, and much remains to be understood about the role played by insects. Although it is undisputed that insects speed the death rate of trees, it is unclear whether they are significant vectors for the pathogen and necessary to kill trees.
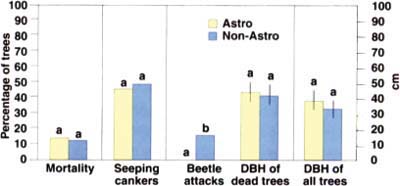
Fig. 2. Comparisons between 67 trees treated with the insecticide Astro and 67 untreated trees in a coast live oak stand. At the end of a 4-month period, new oak mortality and the number of trees with seeping cankers were not significantly different between the two treatments. Data from the two treatments were compared with either chi square or t-test analyses. Different letters indicate statistical significance at P = 0.05. DBH = diameter at breast height.
Although vectoring by beetles or other insects cannot be excluded at this point, our observations indicate that many cankers are initiated without any sign of beetle activity. There are also many cases in which small trees, although unsuitable for colonization by such insects, are infected by the new Phytophthora species and die, again suggesting a secondary role played by insects in SOD.
Marin County field study
In March 2000, 67 coast live oaks were sprayed with the insecticide Astro (36.8% active ingredient permethrin), according to label directions, on the lower 12 feet (4 meters) of tree boles on a Marin County ranch heavily affected by SOD. Only live trees (both healthy and symptomatic) were treated and marked. Trees with extremely thin crowns and abundant signs of beetle activity were regarded as dead and therefore excluded from treatments.
In July 2000, we returned to the site and performed a comparative evaluation of the 67 treated trees (Astro group) versus 67 untreated trees (non-Astro group) in an adjacent stand. None of the trees in the Astro group displayed signs of beetle activity on the treated portions of the boles, while 11 (16%) of the non-Astro trees showed symptoms of beetle attacks (fig. 2).
The two groups were well suited for a comparison:
The overall mortality at the study site (that is, including all 134 trees from the Astro and the non-Astro groups) was approximately 13% (17 individual trees). The number of seeping cankers showed that the levels of infection were not different between Astro-treated and nontreated groups (fig. 2). A total of 9 trees (13%) in the Astro group were dead, despite the notable absence of bark beetles and ambrosia beetles in the treated portion of the trunk. All of the dead trees in the Astro group were mature, and had been killed without the presence of bark beetles. However, all dead trees displayed Phytophthora-caused bleeding cankers, providing strong support of Phytophthora as the primary causal agent of SOD. Furthermore, levels of mortality were not significantly different between the two groups (fig. 2), providing further evidence that insects were not the primary cause of SOD-linked mortality. Finally, all trees in the non-Astro group attacked by beetles (11) also had bleeding cankers, but 22 other trees displayed bleeding cankers without signs of beetle activity. This result reinforces the observation that cankers precede beetle attacks, and that fungal colonization is a factor predisposing trees to attacks by bark and ambrosia beetles.
Both inoculation tests and this beetle-exclusion study point to Phytophthora as the most likely primary agent of SOD in California. Nevertheless, secondary agents including insects and other pathogens are bound to play a significant role. For instance, it has been reported that insecticide applications delay tree mortality for several months (Svihra 2000). This information clearly indicates that insect attacks strongly contribute to accelerated tree death. Our current challenge is to understand the basic biology of the SOD Phytophthora in relation to the plant hosts and to the other agents involved in SOD. An effective control strategy will be feasible only when the sequence of possible interactions among all damaging agents is better understood.
N.M. Kelly is Cooperative Extension Specialist, Department of Environmental Sciences, Policy and Management, UC Berkeley. Kelly is co-director of CAMFER and chair of the COMTF monitoring committee
Department of Environmental Sciences, Policy and Management, UC Berkeley, UC BerkeleyB.A. McPherson is Postdoctoral Researcher, Department of Environmental Sciences, Policy and Management, UC Berkeley. McPherson is a member of the COMTF monitoring committee.
Multi-scale approaches taken to SOD monitoring
As of January 2001, sudden oak death (SOD) had been confirmed by laboratory isolations in six coastal counties, and reported in several others. Early estimates of tree loss indicate that 40% to 80% of the oaks or tanoaks in some areas are affected and likely to die.
Establishing the baseline conditions of SOD and monitoring its spread over the next 5 to 10 years will be critical. The California Oak Mortality Task Force (COMTF) was established in August 2000. One of its goals is to determine appropriate methods for monitoring the SOD epidemic. The task force's monitoring committee is pursuing multi-scale strategies and approaches, utilizing numerous data sources. Researchers with UC Berkeley's Center for the Assessment and Monitoring of Forest and Environmental Resources (CAMFER) are taking the lead in the monitoring effort.
Regional-scale monitoring.
We are using regional-scale monitoring to determine the overall extent and range of SOD; roadside and aerial surveys are helping to determine its geographical extent. Trained field staff visited the sites of reported extensive oak mortality to take samples of affected trees, prior to lab confirmation, in order to determine the most likely current zones of infestation. To date, sampling from areas outside these zones has not revealed additional areas of SOD.
We are also gathering information about individual symptomatic trees using the OakMapper Web site. This site, developed by CAMFER, uses WebGIS (geographic information systems) technology to allow interactive map browsing and submission of the location and condition of trees. This is a valuable tool in developing the regional picture of SOD. Areas where SOD is confirmed can be more intensely sampled, guided by new submissions of oak mortality.
Landscape-scale monitoring.
The conditions and spatial patterns of dead and dying trees are crucial indicators of disease progression. Patterns of spatial variation of the syndrome's progression through a stand can serve as a key diagnostic tool, illuminating possible mechanisms of transmission among trees and interactions among causal agents. For example, clumped distributions could indicate that the disease is spread through roots or elevated insect populations, rather than soil-related or climatic effects. Spread that follows topography would indicate the predominance of root infections via movement of moisture through soil. An increasingly sparse spatial pattern, or a pattern coinciding with hiking trails or hoofed wildlife corridors, might indicate the importance of human or wildlife vectors.
Remotely sensed data (primarily reflected light in the visible and near infrared portions of the spectrum, integrated over a discrete area) is a valuable tool for determining the condition and pattern of trees with SOD. Airborne Data Acquisition and Registration (ADAR) imagery was acquired for two study areas in Marin County by Positive Systems, Inc. Digital imagery was acquired at 1 meter ground spatial resolution in blue, green, red and NIR light. While the imagery is still being processed, preliminary results indicate that in the hardwood forests of China Camp State Park in Marin County, this data can discriminate between dead and dying trees and determine spatial patterns. The resulting maps can be linked to fire spread models. This type of work should be employed in other locations, particularly where SOD is in the early-infection stage. Because tanoak is largely an understory species, there may be some problems identifying the condition and pattern of dead trees in some areas.
Local-scale monitoring.
We established research plots in Marin County to follow symptomatic and healthy trees. Plots were established in March and April 2000, in China Camp State Park (CCSP), near San Rafael, and in Marin Municipal Water District (MMWD), a protected watershed adjacent to Mt. Tamalpais. These plots were selected to encompass a diversity of habitat types and tree species. Coast live oaks and California black oaks grow in both sites, but tanoaks are only found in MMWD. Ten plots were established in each of these sites, for a total of 20 plots and approximately 750 trees.
All oaks and tanoaks greater than 2 inches dbh (diameter at breast height) are included in the survey. Each tree is evaluated for seeping, bark beetles, Hypoxylon fungus and foliage appearance. Plots are evaluated at 2- to 3-month intervals.
Classified ADAR imagery, draped over topography. Red areas are dead tree crowns. Image produced by Kalliopi Tzivanaki of UC Berkeley Department of Environmental Sciences and Policy Management.
Considerable between-plot variation was found in infection rate and mortality at both CCSP and MMWD. Through December 2000, the between-plot range of coast live oaks exhibiting seeping was 8% to 95% (CCSP) (35% average) and 3% to 56% (MMWD) (21% average). For tanoaks in MMWD, the between-plot range was 18% to 80% (55% average). Estimates of mortality in the plots are limited to trees that show clear evidence of seeping cankers prior to death. For coast live oak in CCSP, estimated mortality across all plots ranged from zero to 21% (12% average). In MMWD, coast live oak mortality across all plots was zero to 32% (7% average). The corresponding values for tanoak were zero to 26% (14% average). No infected valley oak were identified.
SOD Web sites
California Oak Mortality Task Force
http://suddenoakdeath.org
Integrated Hardwood Range Management Program (IHRMP)
http://danr.ucop.edu/ihrmp/
UC SOD research team updates
http://camfer.cnr.berkeley.edu/oaks
OakMapper
http://camfer.cnr.berkeley.edu/oaks/map-intro.htm
(Interactive Web-based GIS distribution maps.)
Confirmed SOD sites
http://camfer.cnr.berkeley.edu/oaks/#distribution.
Distribution graphics
http://camfer.cnr.berkeley.edu/oaks/#maps
(Downloadable maps including current SOD distribution and oaks at risk.)
Remote-sensing results
http://camfer.cnr.berkeley.edu/oaks/ADARinfo.html
(ADAR image analysis of two study areas in Marin County.)
Marin County UC Extension
http://cemarin.ucdavis.edu
We are continuing monitoring at all scales, including evaluating satellite remote-sensing at the regional scale, more high-resolution mapping at the landscape scale, and statistically valid field surveys at the local scale. We are hopeful that these efforts will make a valuable contribution to ongoing research in order to find a solution for SOD.
Integrated management approach
We have just begun to unravel some of the dynamics of SOD, and research and outreach professionals are hard pressed to provide control strategies that may lead to disease containment or remission. The three main issues of disease management pertain to our ability to (a) prevent further spread of the disease; (b) contain disease intensity where it is already present; and (c) identify treatments capable of arresting or remitting disease development on infected trees.
Even though cures have rarely been developed for tree diseases, it is not unrealistic to envision a combination of cultural and chemical treatments that may prove effective against the spread of SOD. The extent of control depends largely on the type of strategies available and on their costs, both economic and environmental. The more targeted and less costly the approach, the broader its application. It is also important to keep in mind that landscape trees may display symptoms identical to SOD, but be infected by Phytophthora species other than the SOD Phytophthora. A precise diagnosis is therefore essential, because other Phytophthora-caused diseases require different control approaches. Furthermore, all SOD control strategies must be accompanied by general practices aimed at enhancing and maintaining a tree's vigor and health.
Diagnostic DNA probes
The first step in addressing SOD is to ensure that the trees or plants are indeed infected by the SOD Phytophthora. The traditional approach is based on culturing the pathogen from the border of active infection. After a week or so, the pathogen can be identified in the laboratory based on its morphological traits. Unfortunately, culturing is not always successful, for instance when cankers become old or contamination occurs. This problem is common to Phytophthora species worldwide. Cases in which the pathogen is present but unculturable are called “false negatives.”
We have now developed DNA probes that will allow us to diagnose the pathogen's presence using Polymerase Chain Reaction (PCR) techniques, applied directly to host plant material. This molecular technique recently confirmed the identity of a fungal culture from rhododendron and its presence in several leaves of the same plant. PCR allows for samples to be processed in just a few hours and does not require a culturable stage of the pathogen, nor that the technician be trained in microscopic fungal morphology. The probe will not cross-react with any other Phytophthora species and will allow us to monitor our woodlands and plant nurseries in a more reliable and efficient way.
Inoculum control
Developing targeted inoculum control approaches such as sanitation and quarantine requires a refined understanding of the disease. In the case of a new disease such as SOD, lack of knowledge results in recommendations that may change as more information on the disease biology and epidemiology becomes available. Our current thoughts on SOD management recommendations are based on our present limited knowledge and on extrapolation from similar diseases, but have yet to be verified by specifically designed research studies.
The SOD Phytophthora grows optimally at 68°F (20°C) and is killed at temperatures higher than 95°F (35°C). This sensitivity to high temperatures may be a limiting factor in the inoculum production and therefore in the spread of the disease. All other species in the genus Phytophthora are favored by wet or moist periods, and it is likely that the same may be true for the SOD Phytophthora. For instance, we have observed that fungal sporangia present in the oozing sap in the late spring and early summer become undetectable during the dry summer months. In culture, the SOD Phytophthora produces abundant chlamydospores. In the case of related pathogens, these thick-walled propagules allow the disease agent to survive in soil, water and possibly wood. Although we still do not know where and for how long the SOD Phytophthora chlamydospores may survive, the most conservative approach would be to limit movement of all media that have been shown to harbor chlamydospores of similar pathogens.
Red frass left by western oak bark beetle beetle boring into phloem and xylem of coast live oak. Western oak bark beetle can be found on trees in later stages of sudden oak death syndrome.
The movement of media that potentially vector the disease (such as soil, wood, water and all horticultural material from infested areas) should be limited, particularly during wet periods when inoculum potential is at its highest and the climate may be most favorable to infection. Similarly, tree pruning and any other activity that may facilitate infection should be planned in hot, dry periods, when inoculum potential is at its lowest.
Given the high levels of local mortality, disposal of dead wood is extremely important. Because moist environments favor all Phytophthora species, treatments leading to rapid wood drying are likely to be beneficial. These treatments should decrease the amount of active inoculum present in the wood and may limit the risk of spreading the disease when transporting the wood. Studies are underway to determine the most appropriate way to treat wood; at present we recommend keeping it on the property where it was cut, to limit inoculum dispersal.
There are inherent costs attached to these guidelines aside from the added direct costs of managing for the disease. These costs range from loss of benefits derived from oak woodlands by the general public or land owners (such as limitation of firewood transport and use or limitation of recreational activities) to interference with the financial well-being of those who depend on oaks for their living (such as commercial plant nurseries located in areas of infestation, arborists and so on). Because of these costs, it is very important to verify through research exactly where and for how long the pathogenic inoculum survives. Although our concern is currently to avoid the spread of the disease to new areas, final long-term recommendations need to be based on the results of such research.
Chemical control
Chemical control approaches are routinely used in agriculture, but they have rarely been successfully adopted in trees, with the significant exception of orchard trees. Nevertheless, two successful fungicide treatments of oak diseases — one of which is caused by a Phytophthora species — (Fernandez-Escobar et al. 1999; Osterbauer et al 1994) are compelling evidence that such approaches may be integral components of a control strategy. Other Phytophthora species have been routinely controlled with the use of both fungicides and compounds such as phosphonates applied as foliar sprays or soil drenches, or injected into the trunks of trees. Almond, oak, avocado and cocoa trees have all shown remission or control of Phytophthora cankers in the medium or long term (Guest et al. 1995). It appears that such chemical treatments may be effective both for preventive and curative approaches (Browne and Viveros 2000).
Left, pale sawdustlike ambrosia beetle frass and seeping bark typical of SOD on coast live oak. Above, an adult ambrosia beetle and frass from tunneling into heartwood. Right, an adult ambrosia beetle.
Several compounds have already been tested and proven effective against SOD Phytophthora in the laboratory. Compounds that performed well in the in vitro tests are currently being used in field experiments. Compounds that may not perform well in vitro, but that are known to be effective in stimulating antifungal responses in planta are also being tested in the field. We believe that early treatment may be a key factor in chemical control of the disease.
Although the benefits of chemical treatments are often uncertain for trees, their costs may be many and far-reaching. The most significant costs include the side effects of any active compound used in the applications. Is the compound targeting only the specific pest or pathogen, or is it affecting many different categories of organisms, including humans? Which organisms does it affect the most? How does it disperse in the environment? How long will it be active against the target species and against other species? Will it accumulate in some organisms? Will the pathogen build up resistance easily or not? If resistance occurs, could it spread to other related pathogens?
These questions highlight the risks associated with chemical control, and prompt us to launch into chemical control strategies with the greatest caution. It is also possible that by prolonging the life of seriously compromised trees without a real remission of the disease, we may increase the frequency of other opportunistic tree pathogens that may affect not only the three oak species susceptible to SOD, but also other tree species. By keeping the tree alive for a longer period, we may also allow a longer availability of Phytophthora inoculum present in the bleeding cankers, which are likely to dry out faster upon tree death.
Future for molecular analysis
We are currently using genetic markers called Amplified Fragment Length Polymorphisms that will scan the whole genome of the organism. These will enable us to determine levels and patterns of genetic variability in populations of the pathogen. This information is essential in addressing important issues regarding the origin and the biology of the organism. The molecular information can be used to determine:
Oak trees that are planted in lawns often get overwatered, which leads to their decline. This type of oak mortality is sometimes mistaken for sudden oak death syndrome.
Potential for integrated approach
We believe that a complete approach, including practices to enhance tree health, early disease detection and targeted chemical treatment, may hold some promise for the control of SOD. Rotation of compounds is important to avoid the rise of resistance among populations of the primary pathogen. Chemical control of secondary SOD organisms may also be important. For instance, insecticide applications may be appropriate to protect trees that are on the threshold of becoming attractive to beetles, to prolong their lives while other approaches are undertaken against the primary SOD pathogen.
Although fungicides may eventually prove useful in managing this disease, the mode of compound delivery and the potential for resistance buildup are serious concerns. The potato industry in the Northwest, for instance, has been seriously impacted by the rise of resistance to the widely used compound metalaxyl. The onset of resistance in P. infestans, the causal agent of late potato blight, was the result of sexual mating between pathogenic isolates from different world regions.
Such a complex integrated approach appears feasible for landscape and garden trees, but unfortunately not for trees in woodland situations. Ultimately, the fate of the oak species affected by SOD will be determined by the levels of disease resistance present in natural populations of these trees. We hope that a significant portion of oak populations will display signs of resistance both to infection by the SOD pathogen and to SOD mortality. For the time being, we recommend that SOD-infected trees not be prematurely cut down. The three oak species affected by SOD depend on resistant individuals to build up their populations, which are being decimated by this new disease.