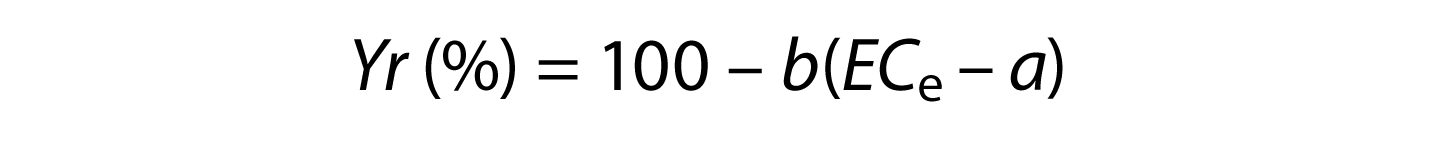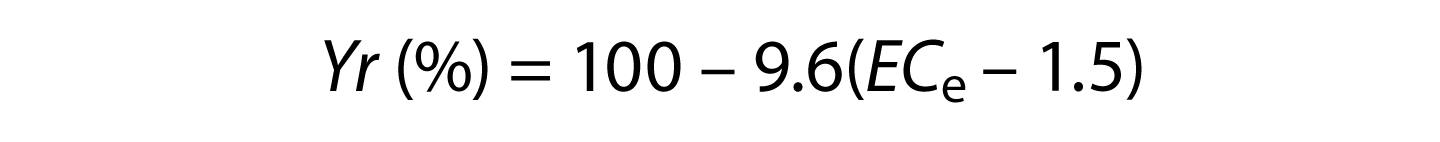All Issues
Recycled water causes no salinity or toxicity issues in Napa vineyards
Publication Information
California Agriculture 68(3):59-67. https://doi.org/10.3733/ca.v068n03p59
Published online July 01, 2014
NALT Keywords
Abstract
In response to Napa Sanitation District's interest in expanding its delivery of recycled water to vineyards for irrigation, we conducted a feasibility study to assess the suitability of the water for this use. We adopted two approaches: comparing the water quality characteristics of the recycled water with those of other local sources of irrigation water, and evaluating soil samples from a vineyard that was irrigated for 8 years with the recycled water. Results indicate that the quality of the recycled water is suitable for irrigation, and also that long-term accumulation of salts and toxic ions have not occurred in the vineyards studied and are unlikely to occur. Nutrients in the recycled water may be beneficial to vineyards, though the levels of nitrogen may need to be reduced by planting cover crops in some vineyards.
Full text
The use of treated municipal wastewater for irrigating crops has increased dramatically in California over the past decade and is expected to expand exponentially in the next few decades (WateReuse 2009). The California Department of Water Resources (DWR) projects that the state's population will grow to 52 million people by 2030 (DWR 2005). Treated wastewater is a necessary water source to meet the needs of this expanding population. In 2009, urban California produced about 9 million acre-feet (MAF) of urban wastewater, of which, surprisingly, only 7% (0.65 MAF) was recycled (WateReuse 2009). The state has set an ambitious goal to increase reuse of wastewater to 2.5 MAF by 2030.
With this goal in mind, the Napa Sanitation District (NSD) has developed a Recycled Water Strategic Plan to explore options to maximize water recycling in Napa County; the plan includes vineyard irrigation, in particular the nearby vineyards in the Carneros region west of the city of Napa and the Milliken-Sarco-Tulocay (MST) region east of the city. Recycling water involves the management and treatment of wastewater to produce water that can be used for irrigation and other beneficial uses (Asano et al. 2007; Vivaldi et al. 2013). Water recycling benefits the environment by limiting the discharge of treated wastewater into natural waterways and helping to preserve the supply of potable water for human consumption (DWR 2003).
A study by UC Cooperative Extension researchers found that vineyards in Napa County irrigated with reclaimed wastewater showed no buildup of salinity or ion toxicity after 8 years.
The production of recycled water is regulated by the California Department of Health Services through Title 22 of the California Code of Regulations, which protects public health while allowing for the safe use of recycled water for agriculture. Wastewater at NSD is treated through a series of primary, secondary and tertiary processes; the steps include settling, biological oxidation, clarification, coagulation, filtration and disinfection. The resulting water is clear and colorless and may have a slight chlorine smell due to the final disinfection treatment (residual chlorine is low enough to meet irrigation water quality standards). NSD's recycled water is “disinfected tertiary quality,” the highest standard for recycled water in California.
Expanding the use of NSD recycled water has many economic and environmental advantages. It provides a reliable source of water to growers who might otherwise have no water or whose supplies diminish late in the summer and during periods of extended drought. The cost of NSD recycled water is generally less than the cost of other sources of supplemental water. Additionally, expanded use of recycled water reduces the amount of wastewater discharge to the Napa River and protects existing sources of fresh water for other uses.
Napa Sanitation District (NSD)
The NSD's National Pollutant Discharge Elimination System (NPDES) permit, issued by the San Francisco Bay Regional Water Quality Control Board, allows for the discharge of treated wastewater into the adjacent Napa River during the wet season (November through April), but during the dry season (May through October) river discharge is prohibited. During the nondischarge period, treated water is recycled for irrigation purposes or stored for wet season discharge. NSD currently delivers recycled water for irrigating vineyards, industrial landscaping and golf courses near the Soscol Water Recycling Facility.
The Carneros region has extensive plantings of vineyards, but water is often limited in this area and there is little surface water available from ponds or reservoirs. Groundwater is often limited in volume, and it may be high in salts or boron, especially from wells close to San Pablo Bay. The MST region includes considerable vineyard acreage and golf courses, which can potentially benefit from the availability of recycled water.
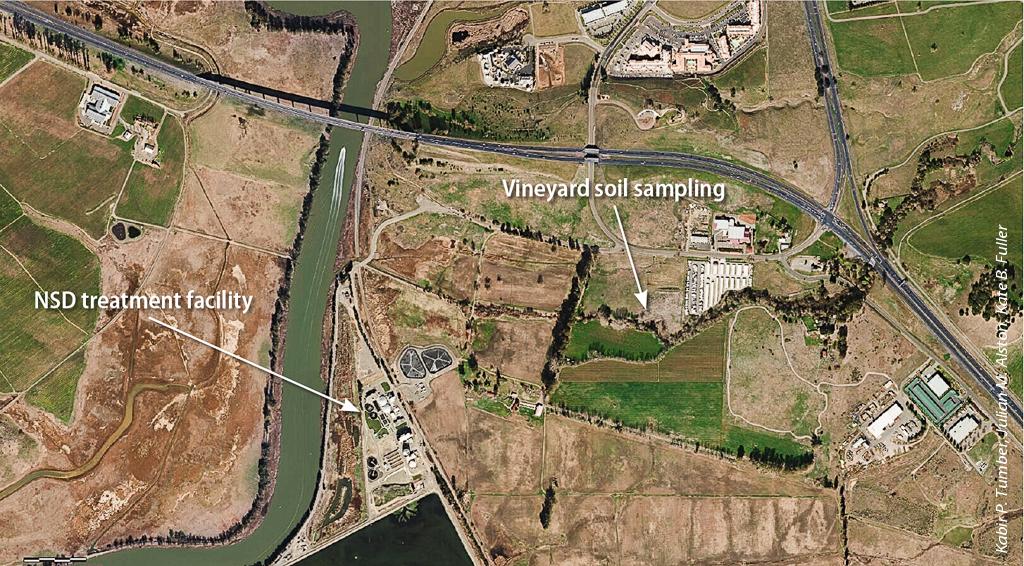
Aerial view of the Napa Sanitation District's recycled water use area and the location of the soil sampling site. The use of treated municipal wastewater for irrigating crops has increased in California in the past 10 years and is expected to expand exponentially in the next few decades.
Study overview
For recycled water to be a benefit to grape growers, it needs to be suitable for vineyard irrigation and there must be no problems with it (such as high salinity or toxic constituents) that could affect the vines or soil; and growers must be confident about its quality and the effects. In 2004–2005, NSD gave UC a grant requesting a feasibility study. For this study, samples of NSD recycled water were collected in 2005 on a weekly basis during the dry season (May 1 through Oct. 31, the period when high-quality recycled water suitable for vineyard irrigation is produced by NSD). Water samples were collected from a 24-hour automatic sampler that maintains a representative sample of recycled water produced at the plant during the previous 24 hours. The sampler collects aliquots every 15 minutes. The quantity collected is based on the flow rate during that period. In this way, a truly representative, composite sample is produced. The samples were collected by NSD staff and sent to Caltest Analytical Laboratory in Napa for determination of key inorganic constituents.
To have water quality data from other local water sources to compare to the NSD samples, we also collected samples from several water sources being used for vineyard irrigation in the Carneros and MST regions. In Carneros, three wells, one surface water storage pond and a domestic tap water source from the city of Napa used for irrigating vineyards were sampled. In the MST region, three wells, one surface water storage pond, and one pond that combined surface water runoff and well water were also sampled. At each of these locations, water samples were collected in May, July and October 2005, that is, at the beginning, middle and end of the dry season, when NSD recycled water is available for irrigation.
Analyses of these samples, similar to the analyses of the NSD samples, were performed at Caltest. To meet Caltest guidelines, water was collected in three containers: one container with no preservatives added, for analysis of alkalinity, chloride (Cl), pH, electrical conductivity (EC), nitrate-nitrogen, nitrite-nitrogen, total dissolved solids (TDS), sulfate (SO4), fluoride (F) and turbidity; another container, with nitric acid as a preservative, for analysis of boron (B), iron (Fe), silica, calcium (Ca), magnesium (Mg), sodium (Na), potassium (K) and hardness; and a third container, with sulfuric acid as a preservative, for analysis of ammonia-nitrogen, organic nitrogen (N), total Kjeldahl N and phosphate. Samples were stored in coolers and transported to Caltest within hours of collection.
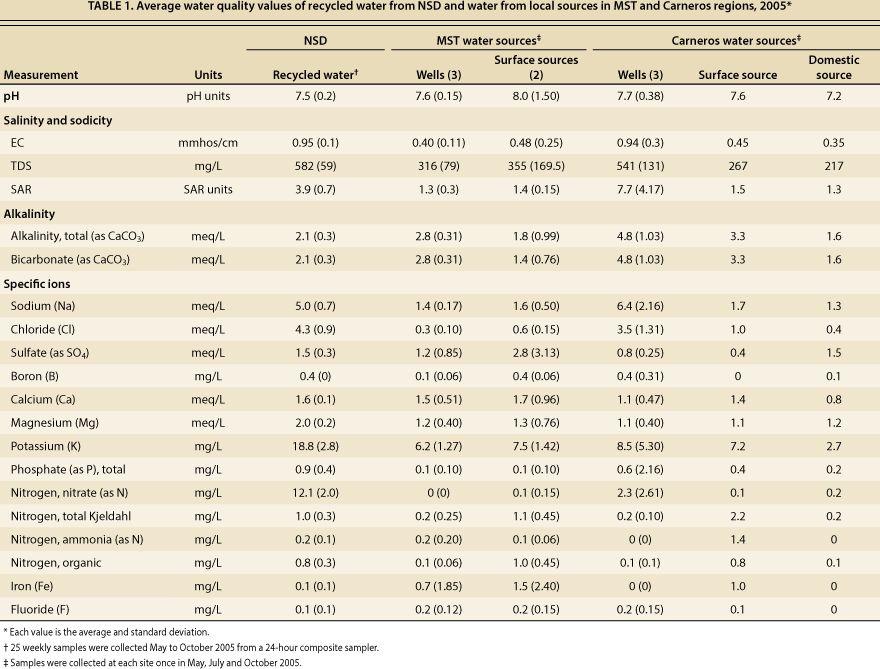
Irrigation water quality evaluations generally consider the water's pH, salinity hazard (which is indicated by the EC of the water and is associated with the total soluble salt content of the water), Na hazard based on the sodium adsorption ratio (SAR, the relative proportion of Na to Ca and Mg ions), alkalinity due to carbonate and bicarbonate ions, and the presence of specific ions such as B and Cl that can have toxic effects and other constituents such as N that can influence plant growth and vine vigor. All of these parameters were evaluated in this study and are presented in table 1.
TABLE 1. Average water quality values of recycled water from NSD and water from local sources in MST and Cameras regions, 2005*
In addition to the water sampling described above, we collected soil samples in September 2005 from a vineyard that had been drip-irrigated with NSD recycled water for eight seasons (1997 to 2005). Soil samples were collected Sept. 15, 2005, at two depths. The grower typically applied 75 to 100 gallons of water per vine per season.
Because the soil samples were collected late in the growing season (but before winter rains occurred), they contained the maximum level of soil salinity likely to be found in the vineyard over the season. Soil samples were analyzed for the electrical conductivity of the saturated soil extract (ECe), saturation percentage (SP), pH, Ca, Mg, Na, Cl, bicarbonate and carbonate. Soil analyses were conducted at UC Davis Analytical Laboratory. Data were analyzed by analysis of variance (ANOVA) using R 2.15.0 software (R Foundation for Statistical Computing); standard error (SE) values were also determined.
Salinity effect on yield
Historically, salinity hazard has been assessed using yield potential as described by the Maas-Hoffman salinity coefficients (Maas and Grattan 1999). According to Maas and Hoffman (1977), as described by Ayers and Westcot (1985), salt tolerance can best be described by plotting relative yield as a continuous function of average root zone soil salinity (ECe). Maas and Hoffman proposed that this response curve could be represented by two line segments: a tolerance plateau with a zero slope, and a concentration-dependent line whose slope indicates the yield reduction per unit increase in soil salinity. For soil salinities exceeding the threshold of any given crop, relative yield (Yr), or yield potential, can be estimated using the following expression:
where a = salinity threshold soil salinity value expressed in dS/m; b = slope expressed in the percentage yield decline per dS/m increase above the threshold; and ECe = average root zone salinity in the saturated soil extract. Note that an ECe value for soil is different than the ECW value for irrigation water. The most up-to-date listing of specific values for a and b, called salinity coefficients, are found in Grieve et al. (2012). For grapes, the a and b salinity coefficients are 1.5 and 9.6. Therefore, for grapes,
Note that when the salinity of the soil (ECe) is less than the salinity threshold for grape (i.e., 1.5 dS/m), then the yield potential is 100%. This indicates that grape yields are not adversely affected by soil salinity until the seasonal average root zone salinity (ECe) exceeds 1.5 dS/m (1 dS/m = 1 mmhos/cm [millimhos per centimeter]).
Leaching, ECw and ECe results
To assess the impact on crop yield of irrigation water with a known ECw, the relationship between irrigation water salinity (ECw) and average root zone salinity (ECe) needs to be known or predicted. This relationship depends on the salinity of the irrigation water (ECw), the leaching fraction and whether the irrigation method is conventional (i.e., surface irrigation) or high frequency (e.g., drip irrigation).
The leaching fraction is the fraction (or percentage) of infiltrated water that drains below the root zone. For example, if 5 acre-inches of water were applied to 1 acre and 1 acre-inch of water drained below the root zone, the leaching fraction would be 0.20, or 20%. Soil salinity is controlled by applying sufficient quantities of irrigation water to leach salts from the root zone. The desired leaching fraction, called the leaching requirement, depends on the salinity of the irrigation water and the crop's soil salinity threshold.
Relationships between ECw and ECe at various leaching fractions under both conventional and high-frequency irrigation systems have been presented by Hanson et al. (2006). These relationships assume that water extraction by roots is proportionately higher in the upper part of the root zone, and even more so with drip irrigation. The relationships also assume steady-state conditions, in which the rate of water entering the soil surface and that draining below the root zone remains constant over time. In the case of NSD recycled water, the average ECw is 0.95 mmhos/cm. Using the high-frequency relationship proposed by Pratt and Suarez (1990) and a long-term leaching fraction of 10%, the formula becomes ECe = 1.35 × ECw. This indicates that soil salinity (ECe) over the long term will not exceed 1.3 mmhos/cm. Because this is lower than the threshold ECe value for grapes (1.5 mmhos/cm), NSD recycled water over the long term should not create salinity problems in vineyards.
This calculation, furthermore, takes no account of leaching by winter rainfall, which reduces soil salinity significantly. Leaching is particularly effective in winter, when vines are dormant and crop evapotranspiration (ETc) is essentially zero. With winter rains averaging approximately 20 inches per year in the Carneros and MST regions, the reclamation-leaching functions provided by Ayers and Westcot (1985) predict that about 80% of salts that accumulate in the top 3 feet of soil can effectively be removed each year through leaching by rain alone. The prediction assumes that the soil profile is replenished with irrigation water before winter rains occur. It is therefore advisable for growers to apply a postseason irrigation in late fall to return soil in the crop root zone to field capacity, so winter leaching is more effective. Postharvest irrigation is already a standard practice in many Napa Valley vineyards if water for irrigation is still available.
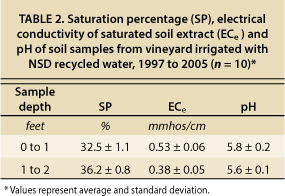
To determine whether there was any evidence of a long-term buildup of soil salinity at the vineyard where recycled water had been applied for 8 years, soil samples from the site were analyzed for ECe and saturation percentage (SP) and the results compared to the threshold ECe for grapevines. Table 2 shows ECe, SP and pH values for an average of 10 samples from two soil depths at two locations (near drip emitters and between rows). The SP values were all similar, which is typical for a sandy loam soil, indicating no major changes in soil texture between the sampling locations. The maximum ECe value among the samples was 0.79 mmhos/cm; most samples were between 0.25 and 0.5 mmhos/cm. No trends with depth or sampling location were evident. The ECe values were all less than 0.8 mmhos/cm, far below the yield threshold of 1.5 mmhos/cm. These field study results provide additional evidence that long-term salinity accumulation should not occur when using NSD recycled water.
TABLE 2. Saturation percentage (SP), electrical conductivity of saturated soil extract (ECe) and pH of soil samples from vineyard irrigated with NSD recycled water, 1997 to 2005 (n = 10)*
Ion toxicity results
Grapevines are sensitive to Cl and to some extent to Na in irrigation water and can develop leaf injury if concentrations exceed certain levels. Specific ion injury, if severe enough, reduces yields more than salinity (i.e., EC or TDS) alone. Although B is an essential element required for plant growth, it is nonetheless potentially toxic, should the concentration in the soil solution become too high. Threshold concentrations of Cl and B in irrigation water, above which toxicity can occur, were reported by Ayers and Westcot (1985) and updated more recently by Grieve et al. (2012).
Chloride.
Many woody species are susceptible to Cl toxicity, with variation among varieties and rootstocks within species. The degree of tolerance is often reflected in the plant's ability to restrict or retard Cl translocation from root to shoots, and particularly to leaves (Maas and Grattan 1999; Walker et al. 2004). Salt tolerance in grapes is closely related to the Cl retention properties of the rootstock, and selection of rootstocks that exclude Cl from scions avoids most Cl toxicity problems (Bernstein et al. 1969).
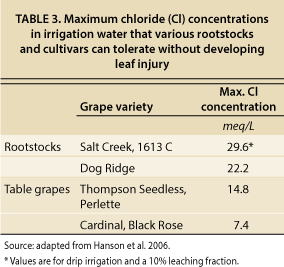
The maximum Cl concentrations in irrigation water that can be used by particular crops without leaf injury are reported in several references cited above, and the guidelines specific to grapes are reproduced in table 3. This list is by no means complete since data for many cultivars and rootstocks are not available, including those currently in use in the Carneros and MST regions. Original data listed by Maas and Hoffman (1977) are in relation to maximal Cl concentrations in the soil water, but data were converted to maximal tolerance in the irrigation water by assuming that EC of soil water is twice ECe and that a long-term leaching fraction of 10% is achieved using high-frequency drip irrigation. These are reasonable yet conservative assumptions.
For sensitive grape cultivars (i.e., Black Rose and Cardinal), the maximum Cl concentration of irrigation water to avoid crop injury is about 7.4 meq/L (milliequivalents per liter) (table 3). Since no tolerance data have been compiled for the predominant grape rootstocks in the Carneros and MST regions (101–14, 5C, 3309 and 110R), we took a conservative approach and selected 7.4 meq/L (262 mg/L, milligrams per liter) as an upper limit for Cl in our study. As more research is conducted on these rootstocks, the limit can be adjusted accordingly. Since the Cl content in NSD water averages 4.3 meq/L (table 1), this water will not likely cause Cl toxicity in grapes, assuming good irrigation water management. If winter leaching is also taken into consideration, the case is even stronger that the recycled water will not pose a problem for vineyard production.
TABLE 3. Maximum chloride (Cl) concentrations in irrigation water that various rootstocks and cultivars can tolerate without developing leaf injury
Sodium.
The ability of vines to tolerate Na varies considerably among rootstocks, but tolerance is also dependent upon Ca nutrition. Much of the early research on Na toxicity was done in the 1940s and ‘50s before the importance was understood of adequate Ca nutrition for maintaining ion selectivity at the root membrane level. Since then, a considerable amount of literature has indicated Na can cause indirect effects on crops, rather than toxicity exactly, either through nutritional imbalances (e.g., Na-induced Ca or K deficiency) (Grattan and Grieve 1999) or by disrupting soil physical conditions (Ayers and Westcot 1985). These indirect effects make diagnoses of Na toxicity per se very difficult. Moreover, Na toxicity is often reduced or completely overcome if sufficient Ca is made available to roots (Ayers and Westcot 1985) through the addition of gypsum or by acidifying soils high in residual lime.
Ca addition reduces the ratio of Na to Ca (Na:Ca) in the soil water, thereby reducing the SAR and exchangeable sodium percentage (ESP), resulting in both improved soil conditions and reduced Na toxicity. Ayers and Westcot (1985) indicate that there are no “restrictions on use” provided that the SAR is less than 3. They provide no concentration limits for Na above which toxicity will result, presumably because of the indirect interactions between Ca and Na mentioned above. The average Na concentration of the NSD recycled water was 5.0 meq/L and the SAR was 3.9 (table 1). Although this Na level is slightly higher than the one suggested by Ayers and Westcot, it can readily be lowered by light gypsum applications in fall. Therefore, these values indicate that Na will not be a problem over the long term provided adequate Ca nutrition and soil physical conditions are maintained.
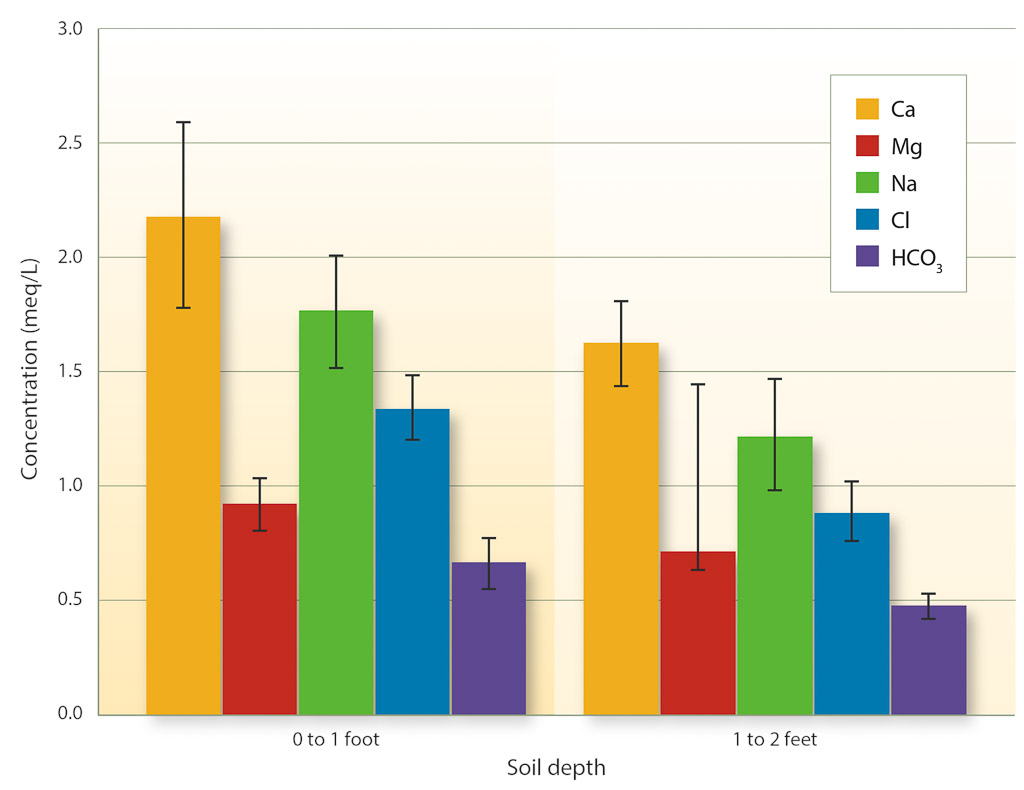
Soil samples collected from the vineyard irrigated with NSD recycled water provide further evidence that toxicities from Na or Cl are unlikely to occur. Figure 1 shows the soluble salts extracted from the soil samples. The average Na and Cl concentrations were 1.6 meq/L and 1.2 meq/L, respectively. Cl toxicity should not be a problem unless the concentration in the saturated soil extract exceeds 10 meq/L (355 mg/L). There is no specific threshold level for Na in soils, as discussed above. The results of these soil tests indicate that toxicities from Na or Cl are not occurring at this site following long-term use of NSD recycled water.
Fig. 1. Chemical constituents in the saturated soil extracts of samples collected in a vineyard irrigated with NSD recycled water from 1997 to 2005 (n = 10). Samples were collected at different depth intervals and distances from the emitter. Bars indicate the standard error.
Boron.
B is an essential element for plants but has a small concentration range between levels considered deficient and those considered toxic. Grapes are particularly sensitive to B in irrigation water and can develop injury to leaves and developing shoots if concentrations exceed certain limits (Camacho-Cristobal et al. 2008). The characteristics of B injury are crop-specific and are related to a plant's ability to mobilize this element (Brown and Shelp 1997). In certain tree species (e.g., walnut and pistachio), B is immobile within the plant, and consequently it does not move out of the leaves once it has accumulated there, resulting in necrosis (burn) along the margins and tips of older leaves. In other tree species (e.g., almond, apricot, apple, nectarine, peach and plum), B is relatively mobile, and injury may not appear first on leaves but instead in young shoots as tip dieback.
Grapevines show some degree of B mobility but not to the same extent as the almond and fruit trees listed above. Threshold levels in irrigation water that produce injury are reported in Ayers and Westcot (1985). Many of these data reported by Ayers and Westcot were taken from Maas and Hoffman (1977), who extracted most of the information, including the grape data, from work conducted by Eaton (1944). When the limited data set from Eaton (1944) is examined in detail, growth of grape does not decline until B concentrations in irrigation water exceed 1 mg/L.
The guidelines for B tolerance are limited. With the exception of a few sand tank studies that provide B coefficients (i.e., threshold and slope) for some crops, most of the B classification work was conducted nearly 70 years ago by Eaton (1944). Research on common rootstocks is lacking. More importantly, the older studies defined a B tolerance limit largely on the basis of the development of incipient injury (i.e., foliar burn) or growth reduction, not on yield response under a range of B concentrations.
The average B concentration of the NSD recycled water was 0.4 mg/L (table 1), which is well below the 1 mg/L level at which grapevines have shown sensitivity. Therefore, it is highly unlikely that B will be problematic over the long term from the use of NSD recycled water. Winter rains will help in leaching soil B below the root zone.
Calcium:magnesium ratios
Some soils in Napa County and parts of the North Coast of California are derived from serpentine parent material, leading to high Mg concentrations (in relationship to Ca), which can affect plant nutrition and reduce plant growth. A review of research studies indicates that plant growth reductions may occur in some plants when the concentration of Mg in soil solution substantially exceeds the Ca concentration (Grattan and Grieve 1999). Levels of Ca and Mg in soil are usually expressed as a percentage of the cation exchange capacity, or on a concentration basis from a saturated paste extract. When comparing concentrations, the levels should be expressed in meq/L.
The relationship between Ca and Mg is often expressed as a Ca:Mg ratio. When Mg is present at three to four times the concentration of Ca (i.e., Ca:Mg ratios of 0.33:1 to 0.25:1), plants, including grapes, often exhibit reduced growth and yield and have low K concentrations in leaves and petioles (R.D. Meyer, personal observation). Ca concentrations may also be lower than desired for normal growth and development. These effects on plant growth often begin to occur when the level of Mg in soil is twice that of Ca (Ca:Mg ratio of 0.5:1).
Adding Ca to serpentine soils (normally in the form of gypsum) can increase the Ca concentration and alter the Ca:Mg ratio. If the Ca:Mg ratio of soil immediately around grape roots is adjusted to a 1:1 ratio or the Ca concentration is adjusted even higher, plant growth will improve and K concentrations in grapes will increase without addition of K fertilizers. High rates of K fertilizer are required to increase the K concentration in plants if Ca:Mg ratios are in the range of 0.5:1. Adding gypsum may be necessary depending on the Ca:Mg ratio and the clay content (greater cation exchange capacity) of soils being used for grape or other crop production.
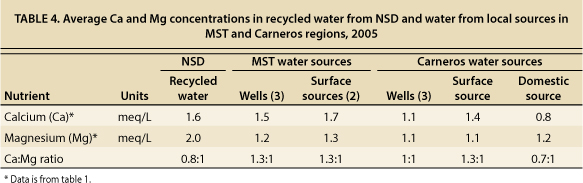
The Ca:Mg ratio of irrigation water is also important because, over time, it may change soil characteristics — if large amounts of irrigation water are applied to soils relative to the amount of rainfall, soil characteristics eventually take on the irrigation water characteristics. MST and Carneros well and surface waters had Ca concentrations equal to or higher than Mg concentrations (table 4). NSD recycled water and Carneros domestic water had Ca concentrations slightly less than Mg concentrations, but they were not so low as to raise concerns regarding the long-term effects on soils. When irrigation waters have at least twice as much Mg as Ca (equivalent concentration bases), then gypsum additions should be made to increase Ca levels in order to keep the soil ratios in balance. The Ca and Mg concentrations in NSD recycled water do not indicate the need for growers to make gypsum additions.
TABLE 4. Average Ca and Mg concentrations in recycled water from NSD and water from local sources in MST and Carneros regions, 2005
Trace elements.
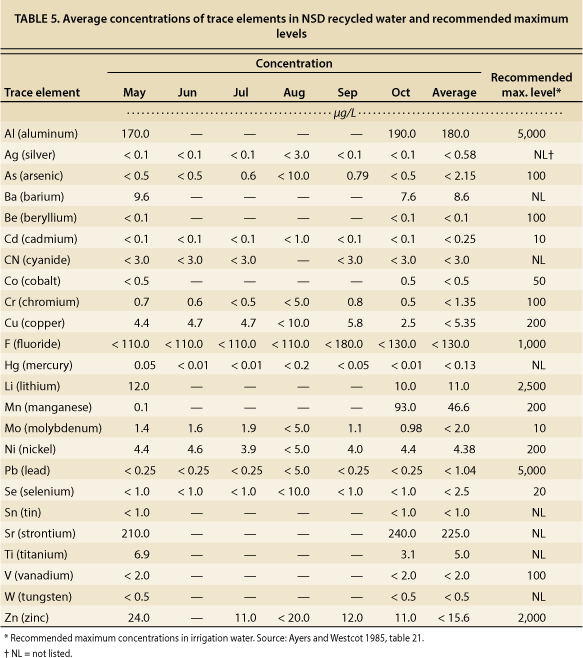
Tests for trace elements, including heavy metals, were conducted on NSD recycled water samples, as required by the NPDES permit. Depending on the element, tests were conducted once a month from May to October, or once in May and once in October. Levels of trace elements in NSD recycled water were well below established thresholds of concern for irrigation water (table 5).
TABLE 5. Average concentrations of trace elements in NSD recycled water and recommended maximum levels
Fertilizer in recycled water
NSD recycled water contains plant nutrients N, P (phosphorus) and K in concentrations that make it a dilute fertilizer solution. Growers should take into account the value of nutrients in reclaimed water and reduce application of fertilizers accordingly, particularly since there is a risk of overapplying N when irrigating with recycled water (Wu et al. 2009). N is the most frequently deficient macronutrient in vineyard soils, and it plays a major role in many of the biological functions and processes of vines, and also of fermentative microorganisms, which can influence quality components in the grape and thus the wine (Bell and Henschke 2008).
Vines that receive fertilizer applications balanced with their needs, left, show no excess vigor. Vines given too much N produce too much vegetation, right, which can lead to reduced yield and lowered wine quality. The N content of recycled water must be taken into account to keep vines in balance.
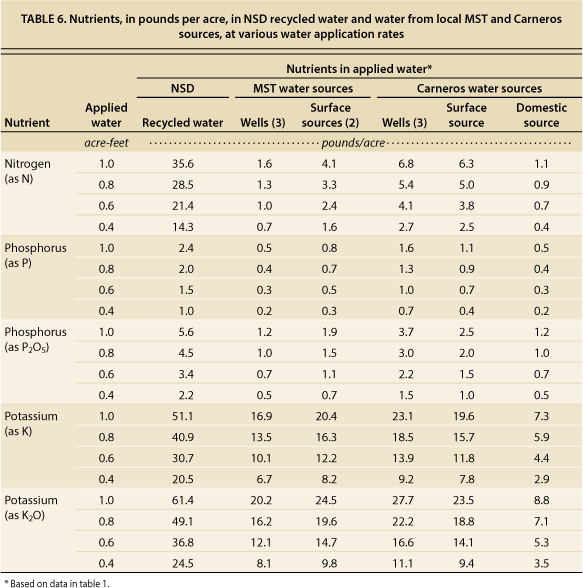
The amount of nutrients delivered to grapevines depends upon concentrations in the recycled water and amount of water applied. Seasonal averages of nutrients in NSD recycled water (table 6) indicated approximately 13.1 mg/L of N (mostly as nitrate-nitrogen), 0.9 mg/L of P and 18.8 mg/L of K. Averages of well and surface waters in both regions were considerably lower: 1.4 mg/L of N, 0.3 mg/L of P, and 7.4 mg/L of K. Table 6 indicates the amount of nutrients in pounds per acre that were applied in NSD recycled water. For comparison, values are also given for MST and Carneros local water sources. At typical irrigation rates of 0.4 to 0.6 acre-feet per acre per season, NSD recycled water delivered approximately 14 to 21 pounds of N, 1 to 1.5 pounds of P (2.2 to 3.4 pounds of P2O5) and 21 to 31 pounds of K (25 to 37 pounds of K2O) per acre. Fertilizer rates for P and K are normally expressed as P2O5 and K2O, respectively.
TABLE 6. Nutrients, in pounds per acre, in NSD recycled water and water from local MST and Carneros sources, at various water application rates
The levels of P and K in NSD recycled water have no detrimental effects on vines; in fact, vines may benefit from application of these nutrients. N is required for proper growth and development of grapevines, but high levels of N can create problems due to excess growth and vigor. Vines with high vigor produce large amounts of vegetation, which takes carbohydrates and sugars away from the fruit and also shades the fruit, which in turn can lead to reduced fruit yields and lowered wine quality. Fruit produced under shaded conditions is likely to be higher in pH, lower in sugar and color, and may have herbaceous characteristics that are undesirable. In addition, high-vigor vines often have a greater incidence of Botrytis bunch rot and powdery mildew diseases.
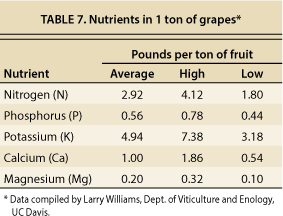
Table 7 shows the amounts of major plant nutrients (in pounds) present in 1 ton of grapes. Assuming a typical yield of 3 to 5 tons per acre, 9 to 15 pounds of N are removed from the vineyard each year with the harvested crop. In comparison, the amount of N delivered in NSD recycled water during the 2005 season was not exceptionally high (14 to 21 pounds per acre), but it may be high enough to be of concern to some growers and wine-makers, especially on sites that typically exhibit vines with vigorous growth. Many vineyards in the Carneros and MST regions are fertilized with N at rates approaching or exceeding these levels, but others are not, or they may not be fertilized with N every year. There are some vineyards that rarely (if ever) receive N additions.
Growers concerned about the additional N supplied with recycled water should consider the use of cover crops to remove the excess N. The choice of cover crop species is important: Legumes fix atmospheric N, which will increase the supply of N to vines and aggravate the problem; cereals and other grasses, which do not fix N, are best grown over the winter dormant period, because they compete with vines for water and nutrients other than N.
Recycled water can be a reliable source of water to growers whose supplies diminish late in the summer and during periods of extended drought. Above, Malbec vines at Trinitas Cellars vineyard, which has been irrigated with recycled water for over 7 years.
Our work here suggests that treated municipal wastewater from the NSD is suitable for irrigation of vineyards over the long term. There was no indication from the water quality parameters assessed that salinity, sodicity or specific ions will limit the use of the water for irrigation. Nutrients in the wastewater can be beneficial, but the N can produce excess vegetative growth in vineyards with high background soil N levels. Ingredients in personal care products and pharmaceuticals are not listed in table 1 and were not evaluated in this study. Although it is unlikely those constituents will be problematic, future research is needed to determine whether they can be accumulated by the vine and transported to fruit tissue.