All Issues
Diagnostics in animal health: How UC helps exclude and minimize impact of livestock pathogens
Publication Information
California Agriculture 68(4):109-116. https://doi.org/10.3733/ca.v068n04p109
Published online October 01, 2014
NALT Keywords
Abstract
UC has a wide reach in the agriculture sector of the California economy and is well recognized for research expertise in plant diseases. Less well known is the role UC plays in animal agriculture. In 2012, the California Animal Health and Food Safety lab at UC Davis performed nearly 980,000 tests on samples from sick livestock, including cattle, horses, pigs, chickens and turkeys. The lab is prepared to respond rapidly to any disease outbreak or identification of a foreign disease. Researchers at the School of Veterinary Medicine at UC Davis are testing novel subunit vaccines to prevent pinkeye in cattle; UC ANR specialists and advisors and the staff at the Sierra Foothill Research and Extension Center were key to the development of best management practices that landowners and resource managers are using to protect their herds and public water sources against the parasite Cryptosporidium parvum; and UC veterinary scientists are part of a large team of experts, including state and federal agencies, determined to combat the endemic bluetongue virus, which can affect the state's exports.
Full text
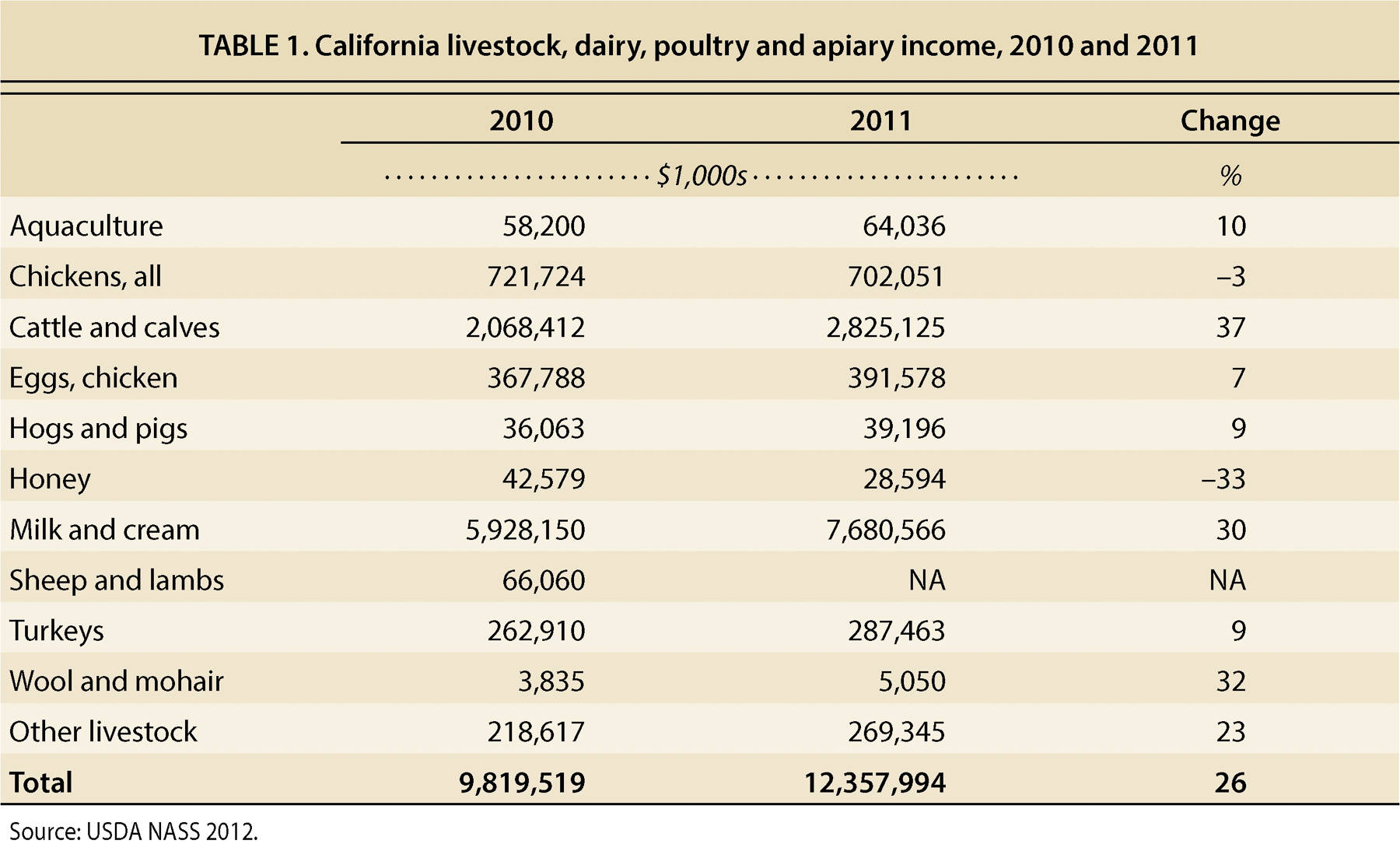
Cash receipts for California's total livestock and livestock products were $12.3 billion in 2011 (table 1). The responsibility for protecting the livestock industries and, by extension, food safety and security, in the state falls on an integrated system that starts on the farm and extends to a wide array of private and public entities. UC has a large role in outreach, education, surveillance and response to significant animal disease events, and faculty and staff at each of the UC campuses are involved.
UC campuses and UC Cooperative Extension work with the CAHFS lab system, funded largely by CDFA, to research livestock diseases, protect animal health and respond to disease outbreaks.
The CAHFS laboratory system
The California Animal Health and Food Safety (CAHFS) laboratory system is funded in large part by the California Department of Food and Agriculture (CDFA) and operates within the UC Davis School of Veterinary Medicine (SVM). CAHFS is the backbone of California's early warning system to safeguard public health from foodborne pathogens, toxins and diseases common to animals and humans. CAHFS also protects the health of California's livestock and poultry populations by providing broad-based surveillance for all catastrophic animal diseases not currently found in the United States (referred to as foreign animal diseases, or FADs).
CAHFS must immediately detect an introduction of a highly contagious disease like avian influenza in poultry and foot and mouth disease in livestock to minimize the devastating effects it would inflict on consumer confidence and California's agricultural economy. As a result, CAHFS is responsible for the vast majority of diagnostic testing of livestock samples within the state. In 2012, the lab system had 30,567 cases submitted to it (4,255 equine, 6,952 bovine, 1,855 caprine, 340 porcine, 819 ovine, 2,064 other mammals, 3,063 chicken, 477 turkey, 1,114 other avian, and 9,805 cases in which a species was not provided, involving feed or bedding samples, etc.) and performed nearly 980,000 tests. The case load that year included over 10,800 necropsies (animal autopsies) of whole animals or animal tissues submitted for full diagnostic work-up (E. Sanson-Smith, CAHFS, personal communication). The work-ups typically include post-mortem and microscopic examination, bacterial cultures, testing for viral infections, testing for antibody levels and, in some cases, testing for the presence of toxic agents.
CAHFS is a member of a number of important national diagnostic networks, including the National Animal Health Laboratory Network (NAHLN) ( www.nahln.org/ ) and the Food Emergency Response Network (FERN) ( fernlab.org/ ). NAHLN is a partnership of federal, university and state veterinary diagnostic laboratories across the United States and is a surveillance and emergency response system that provides critical and ongoing resources for disease surveillance testing, information management, quality assurance and the development and validation of new diagnostic tests. FERN is also a federal-state partnership and provides critical and ongoing resources for responding to microbiological, chemical or radiological food contamination incidents, for which the U.S. Department of Agriculture (USDA) and the U.S. Food and Drug Administration (FDA) have regulatory authority.
How samples get to the labs
The primary route by which samples get to CAHFS labs is through submissions by private veterinarians, owners and state and federal regulatory authorities (fig. 1). This widely diverse group of people ensures that the surveillance is broad based from both geographical and industry perspectives. The laboratory system has four locations in the state: Davis, Turlock, Tulare and San Bernardino ( cahfs.ucdavis.edu/ ).
Fig. 1. The route that samples from a diseased animal and the subsequent case follow through the California Animal Health and Food Safety (CAHFS) laboratory system.
The Davis laboratory is adjacent to the SVM complex on campus and is the largest of the four laboratories. The Davis lab receives a variety of avian and mammalian livestock samples for diagnostic evaluation and is the site for both the CAHFS toxicology section and the equine drug testing program. The toxicology section is nationally recognized and has partnerships with a variety of federal agencies, such as the Federal Bureau of Investigation (FBI), the FDA, and the Centers for Disease Control and Prevention (CDC). Faculty and staff in this section routinely test for toxins such as oleandrin and various rodenticides as well as natural substances such as nitrate, copper and lead.
The Turlock laboratory performs testing on avian samples and was instrumental in the identification of very virulent infectious bursal disease (vvIBD) in the state in December 2008. This disease, by infecting an organ important in the immune response in commercial poultry, can cause severe morbidity and mortality. The disease has been present in Europe for a number of years but, until it was recognized by the diagnosticians in Turlock, had not been reported previously in the United States (Pitesky et al. 2013).
The Tulare lab works with both mammalian and avian species and has had a significant role in recent identification and eradication efforts directed toward Mycobacterium bovis (bovine tuberculosis) in a small number of dairy herds in California.
The San Bernardino lab also works with both mammalian and avian species and was instrumental in the diagnosis and eradication efforts directed at the exotic Newcastle disease (END) outbreak in 2002 to 2003 in Southern California (Nolen 2002). The original diagnosis of this economically important disease was made on a backyard chicken that had been referred to the San Bernardino lab by a local small-animal practitioner. Eventually the effort to control and eradicate this disease from California involved nearly 1,700 personnel from numerous agencies and a total cost to the state and federal governments of $168 million over 10 months (E. Sanson-Smith, CAHFS, personal communication). The CAHFS San Bernardino branch served as the central entry point for birds that were necropsied on-site and for samples that were shipped to the USDA National Veterinary Services Laboratory and the Davis CAHFS lab for testing for the presence of the causal virus.
The CAHFS lab in San Bernardino was instrumental in the diagnosis and eradication efforts during the 2002–2003 outbreak of exotic Newcastle disease, which was originally diagnosed in a backyard chicken.
Research into livestock diseases
Research conducted within UC, including the Agriculture and Natural Resources (ANR) network of advisors, specialists, and UC Davis College of Agricultural and Environmental Sciences (AES) faculty and the staff at the many ANR Research and Extension Centers (RECs), is vitally important in helping the state's producers control endemic diseases of livestock, safeguard the microbiological safety of food and water and improve the sustainability of production agriculture.
Pinkeye.
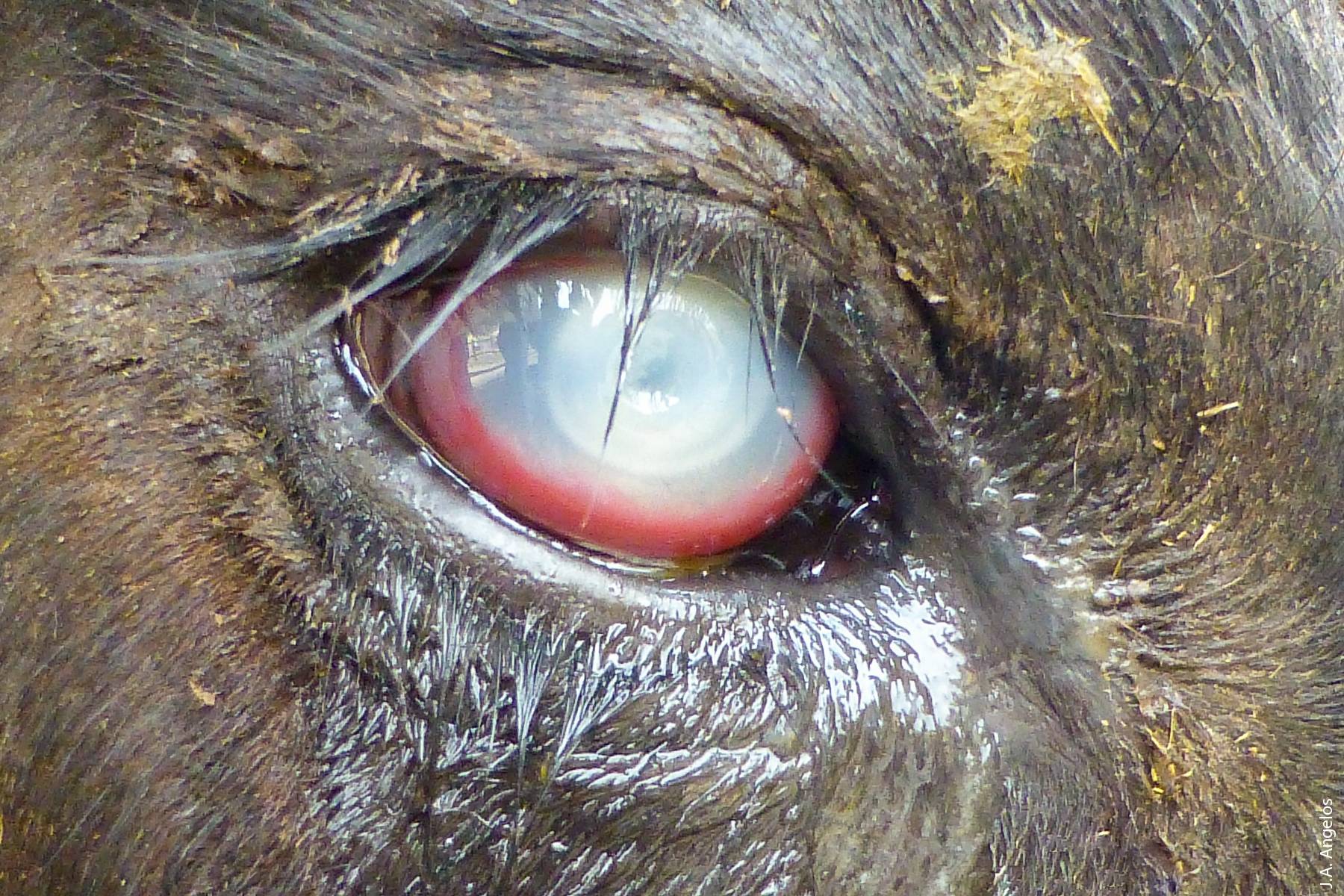
One disease that affects both beef and dairy cattle statewide is pinkeye, also known as infectious bovine keratoconjunctivitis (IBK). IBK is the most common eye disease of cattle worldwide (Angelos 2009). Compared to human pinkeye, bovine pinkeye is a much more devastating disease as it affects not only the conjunctiva surrounding the eye but also the cornea itself, and in severe cases causes blindness following rupture of the eye. IBK is a multifactorial disease, meaning that many different factors contribute to its development, including environmental conditions (plant awns such as foxtails, dust, ultraviolet light), insects (flies), bacteria (Moraxella bovis) and viruses (Angelos 2009).
Bovine pinkeye, the most common eye disease of cattle worldwide, affects not only the conjunctiva surrounding the eye, but also the cornea itself, and in severe cases causes blindness.
Dr. John Angelos, an SVM professor, is building on the earlier work by Lisle George (retired) in conducting research into the pathogenesis and prevention of this important disease. Their collective work has helped characterize important pathogenic factors associated with Moraxella bovis and identified genes encoding these factors (Angelos et al. 2001; Angelos, Ball et al. 2007). In addition, they have discovered Moraxella bovoculi, another Moraxella species believed to play a role in bovine pinkeye (Angelos, Spinks et al. 2007). Current research efforts are directed at developing and testing novel subunit vaccines as well as testing alternate routes of vaccination such as intranasal vaccines to prevent IBK (Angelos et al. 2010, 2012). Collaborative efforts between SVM and UC Davis Department of Animal Science faculty and UC Cooperative Extension (UCCE) personnel and ANR resources such as the Sierra Foothill Research and Extension Center (SFREC) have greatly enhanced research into the pathogenesis and prevention of IBK under naturally occurring conditions typical for California's cattle industries.
Cryptosporidium parvum.One of the leading waterborne infectious diseases that is transmitted between animals and humans throughout the United States is the protozoal parasite Cryptosporidium parvum. Following several community outbreaks of this disease in the 1990s, concerns escalated by regulatory agencies and drinking water districts that livestock were loading watersheds with this parasite resulted in ad hoc grazing restrictions on private and public rangeland watersheds (Atwill et al. 2012). To clarify the processes causing this emerging human health risk and to develop practical farmlevel solutions for the state's livestock industry, a large collaborative effort was initiated in the late 1990s by ANR specialists, advisors and faculty at UC Davis. Key members of the collaboration included Rob Atwill, SVM; Ken Tate, Thomas Harter and Randy Dahlgren, AES; UCCE advisors and their staff throughout California (e.g., livestock and natural resources, watershed, and dairy specialists); state and federal agencies; local water districts; private landowners; and livestock and agricultural organizations such as Backcountry Horsemen of California, California Cattlemen's Association and the California Wool Growers Association.
The research team conducted a series of epidemiological studies in livestock herds on the risk factors for animal infection and also DNA fingerprinting projects to investigate which species of Cryptosporidium were being shed by wildlife and livestock and whether these strains were infective for humans (Rochelle et al. 1999; Xiao et al. 2002). Perhaps more importantly, the team conducted a series of experimental soil box trials at the Veterinary Medicine Teaching and Research Center, SVM, followed up by a set of detailed field trials at the SFREC and the San Joaquin Experimental Range, USDA Forest Service, that studied the efficacy of vegetative buffers to remove waterborne pathogens from rangeland runoff (Atwill et al. 2002).
The collaborations on this disease research have created one of the largest and most integrated scientific teams in the United States working on waterborne pathogens in agricultural watersheds. The results are a variety of practical and adoptable best management practices and science-based tools that livestock owners and resource managers can use to reduce the risk of waterborne pathogens from livestock grazing (Atwill et al. 1999). The efforts of ANR, ranging from the specialists and advisors to the staff and resources at the SFREC, were key to the development of these best management practices, which are widely adopted today by both landowners and resource managers.
Lake Kaweah in the Sierra foothills of California, one of the watersheds in which researchers studied comanagement of livestock grazing and water quality.
Bluetongue.
Bluetongue (BT) is a non-zoonotic disease of certain wild and domestic species of cloven-hoofed ungulates with substantial adverse economic impact on livestock production in California. The causative agent of BT, bluetongue virus (BTV), is an arbovirus that is spread through temperate and tropical regions of the world by biting Culicoides midges, which serve as biological vectors. There are several different serotypes of the BTV, and the individual serotypes may be endemic or newly introduced; the newly introduced serotypes often cause outbreaks of disease in naïve animals.
Bluetongue disease, an arbovirus that affects sheep, cattle and other cloven-hoofed ungulates, is spread by a biting midge (Culicoides sonorensis) and is an ongoing cause of economic loss to livestock producers in California. UC researchers are part of a collaborative effort to conduct bluetongue virus infection prevalence studies and to develop predictive models.
The global distribution of BTV infection has recently altered, likely driven in part by climatic influences on the midge vectors resident in different regions. Similarly, the behavior of BTV infection of livestock has recently altered, with an alarming apparent increase in the occurrence of overt disease in infected cattle (MacLachlan and Mayo 2013). BTV has long been endemic in California and is an ongoing cause of economic loss to livestock producers, with both direct losses caused by reduced production of infected animals and, even more importantly, the adverse impact of nontariff trade restrictions, which close lucrative potential export markets.
Researchers within the UC system target bluetongue in a collaborative effort involving individuals at SVM, UCCE, UC Riverside, CDFA, CAHFS, USDA, and also local veterinarians and local cattle and sheep producers. These extensive interactions and collaborations are critical to the intensive surveillance studies that underpin ongoing efforts to develop accurate and predictive mathematical models relevant to this economically important arboviral disease of livestock. The research group, led by Dr. Jim MacLachlan, has an extramurally funded (currently by NIFA USDA) project to further characterize the ecological drivers of BTV infection among Culicoides vectors and ruminant hosts in the state. The long-term objective is to utilize data generated by the studies for the predictive modeling of this emerging and economically important arboviral infection.
The group's specific objectives are to perform spatial BTV infection prevalence studies to identify environmental, climatic and land-use (and anthropogenic) characteristics associated with regional (local-scale) BTV infection rates of livestock in different regions of California. Initial studies were focused on intensively farmed dairy cattle, and the group is expanding its investigations to free-ranging cattle and sheep. The studies also include extensive entomological investigations to better define the impact of climatic and anthropogenic drivers of the population dynamics of vector midges, and their subsequent impact on the dynamics of BTV infection. Lastly, the group is studying the feeding behaviors of the vector midge species that might be important virus vectors in California and genetically characterizing the evolution of the types of BTVs that circulate in the state, including, most recently, a newly incursive novel virus serotype.
Outreach to producers
UCCE has 23 livestock advisors spread across the state ( ucanr.edu/sites/UCCE_LR/Beef_Cattle/Beef_Cattle_Advisors/ ). They play an important part in the animal disease network, serving a role in education and applied research with livestock producers in both the management of endemic diseases and in the surveillance for foreign animal diseases. They are supported by three statewide veterinarian specialists, who provide a conduit for information from the campus to livestock producers. This network of advisors and specialists also provides identification of diseases occurring in the field based on observed clinical signs. The CAHFS laboratory system then confirms these observations with laboratory findings. The link provided by UCCE personnel between producers and CAHFS is also used to inform producers about any new diseases in their area. UCCE works with producers and their veterinarians on management actions they can take to minimize the occurrence of the disease in their herd. Applied research may also be required with laboratory analysis to determine the effectiveness of the mitigating management action.
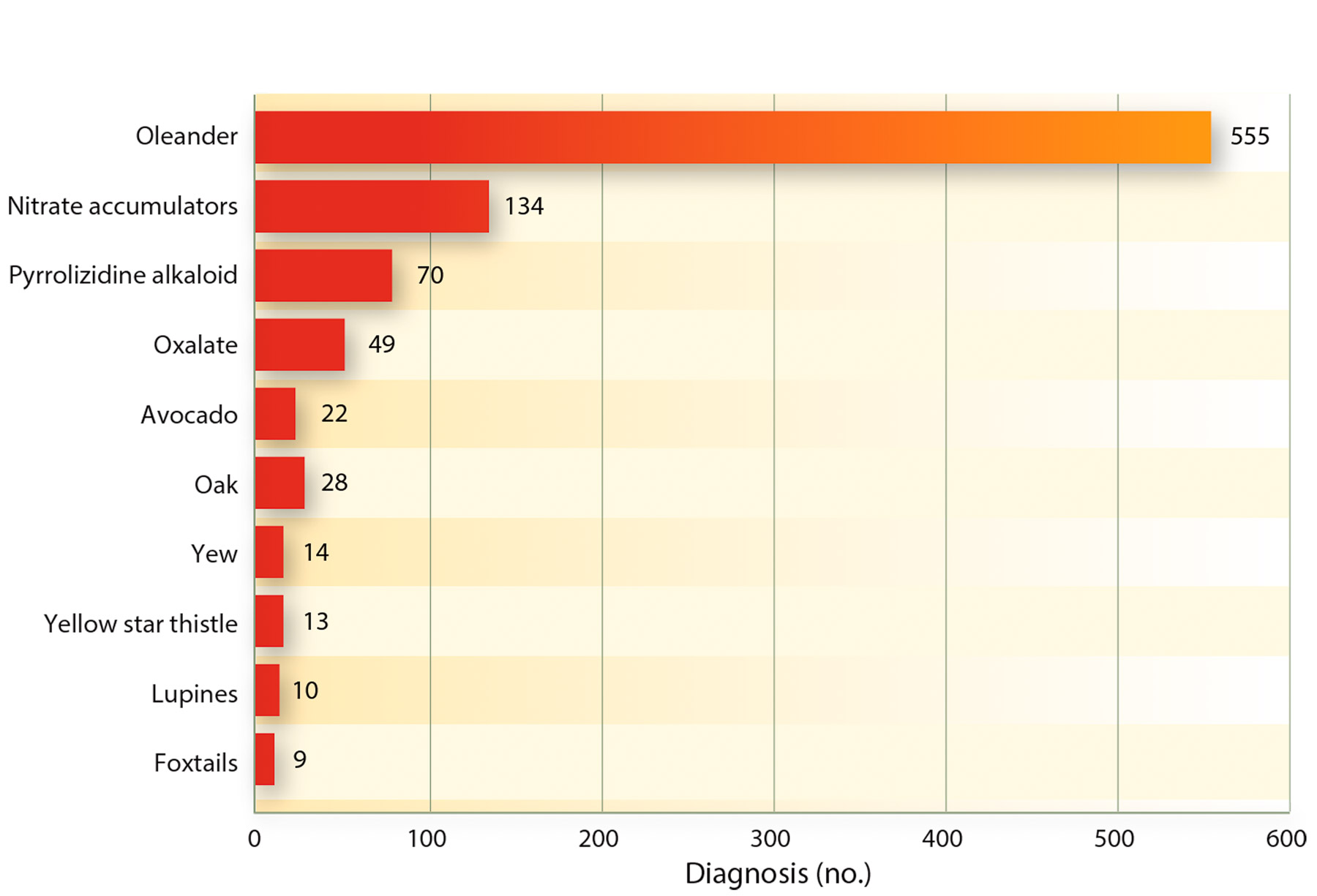
Recently, the CAHFS laboratory database of toxicological findings was mined for use in a UCCE publication on poisonous plants in California (Forero et al. 2011). It reported that for all kinds of livestock the number one reported problem from toxic plants was oleander, with over 555 cases during 17 years (fig. 2). Although this plant is used by Caltrans and others because of its drought hardiness, it does have an impact on the animal population, which needs to be highlighted. The publication by Forero et al. (2011) and the information provided also helped producers define the risk of plant poisonings.
The CAHFS laboratory system and UCCE also play an important role in poultry diseases. Many backyard poultry operations allow their birds to roam free and intermix with wild birds, and as a result they represent a potential portal for the transmission of diseases such as AI from wild birds. Due to the relative lack of veterinary clinics with poultry expertise, many backyard producers look to UCCE livestock advisors to help them with routine management and disease identification during a disease outbreak. This partnership between Extension personnel and the CAHFS lab system is an important part of the effort to protect both commercial and noncommercial poultry as well as the general public from diseases originating in wild birds.
The CAHFS laboratory system and UCCE specialists have worked together to define and test new diagnostic procedures that have higher accuracy or shorter turnaround times. For example, sampling of bulls to test for trichomoniasis, a venereal disease of cattle, requires that the animals be gathered and restrained in a chute. When culture-based methods were in use, bulls had to be sampled three times to be certain they were not infected, which led to increased feed costs and potential trauma to the animals from repeated handling. In response to requests from the California Cattlemen's Association, the CAHFS lab system adopted a PCR-based test that had originally been developed by Dr. Bob BonDurant of SVM (Ho et al. 1994). It has subsequently been determined that the same degree of certainty that an animal is free of infection can be provided by testing a single sample via PCR as was formerly gained by culturing three separate samples. This has resulted in a faster test turn-around time and significantly less handling of bulls.
UC continues to demonstrate its value on a daily basis by playing an important and wide-ranging role in the protection of animal agriculture, by being heavily involved in the surveillance for and diagnosis of important diseases of livestock, by performing fundamental and applied research on those same diseases and by distributing the knowledge gained back to producers. UC is also an important source for expert knowledge about livestock diseases to both federal and state regulatory officials on a regular basis and during disease outbreak emergencies.
Solving the puzzle of foothill abortion in beef cattle
Authors
Author Affiliations
Text
Foothill abortion, also known as epizootic bovine abortion (EBA), has been a long-standing problem for California beef cattle producers. It is a major source of economic loss for California cow and calf producers, and in the 1990s it was estimated that 5% to 10% (45,000 to 90,000 calves) of the California beef calf crop may be lost each year (Bushnell et al. 1991). UC Cooperative Extension (UCCE) farm advisors, specialists and UC Davis School of Veterinary Medicine (SVM) faculty have worked on this disease for nearly 50 years. This long research process finally moved forward in 2005, when the causative agent was identified.
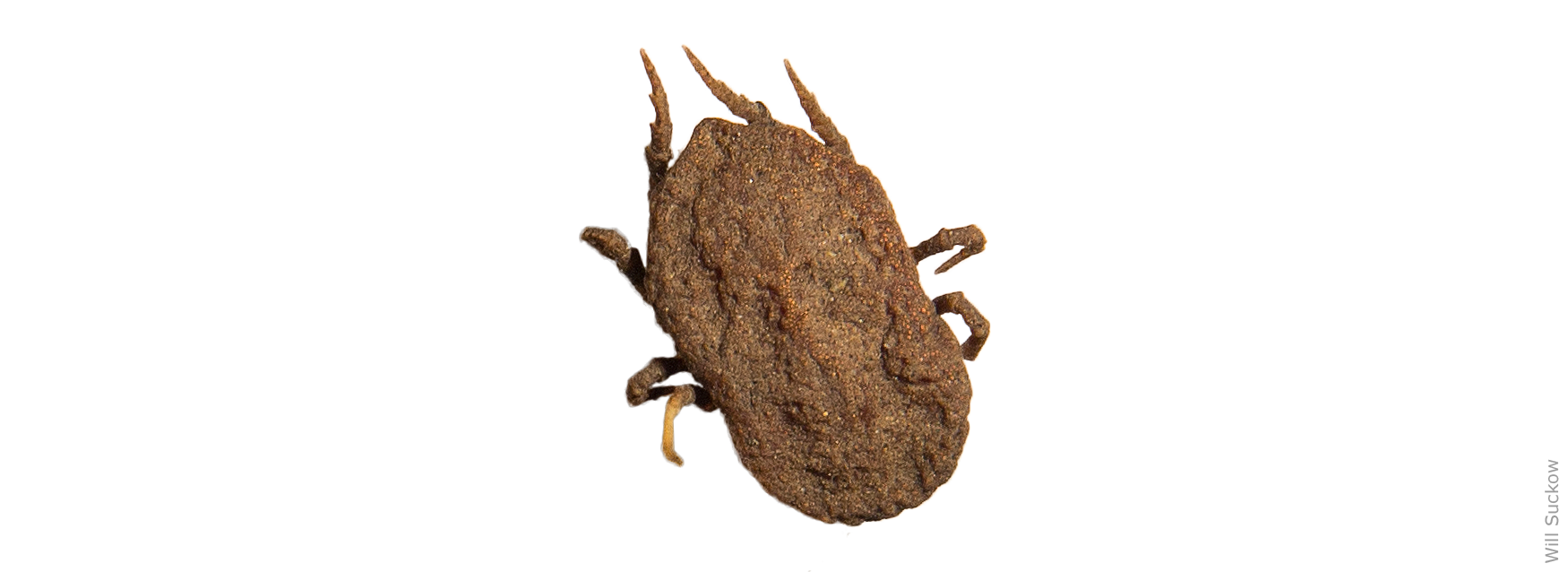
The Pajaroello (pa-ha-WAY-lo) tick, Ornithodoros coriaceus, is responsible for transmitting the causative agent (a deltaproteobacterium) when it feeds on a pregnant cow. The Pajaroello is a soft-bodied tick that resides in dirt or litter under trees and bushes, locations where deer and cattle typically bed down. The Pajaroello does not embed itself in animal flesh, but rather it feeds rapidly (for as little as 20 minutes) and then drops back onto the ground. It can survive for years in a dormant state, without taking a blood meal.
If a cow or heifer is bitten by a tick when 2 to 6 months pregnant, the calf may abort or be born weak. Heifers and cows that have not previously grazed in tick-infested pastures are most susceptible. Once bitten, cows appear to gain some degree of immunity, but ranchers have observed that immunity can be lost if cattle go for a year or more without tick bites, which serve as an immunity booster.
Early UC efforts focused on identifying the vector of the disease. First, mosquitoes were suspected. They were eliminated as a possibility when cattle elevated off the ground (in an area where the disease commonly occurred) carried their calves to term. Additional experiments also eliminated the Leptoconops gnat as a possible vector.
It was initially thought that the Pajaroello tick did not live in the most northern areas of California, where EBA occurred. When ticks were subsequently trapped on a northern Lassen County ranch that had experienced abortions, it was confirmed as a potential vector. Ticks were collected and placed to feed on susceptible heifers (on the UC Davis campus), and abortions occurred, confirming the Pajaroello as the vector of the disease.
The next step was to determine the causative agent being transmitted by the bite of the tick. This was difficult because the tick harbors numerous potential causative agents. In the late 1960s and through the 1970s, chlamydia was considered as a possible causative agent. During this period, a field trial was conducted with cooperator cattle in Lassen County in which susceptible heifers were fed tetracycline crumbles, and the data suggested there might be some protection from the antibiotics. Numerous chlamydia vaccines were prepared and given to susceptible heifers, but this effort was ultimately abandoned when heifers continued to abort following vaccination.
In the 1970s and early 1980s, viruses were considered as possible causative agents. A large research effort was initiated, with over 80 viruses isolated from the tick. After exhaustive work, research on causative agents moved from viruses to spirochete-like organisms; a Borrelia species was suggested to be a potential cause of EBA, but further experimental studies essentially eliminated spirochetes and Borrelia species as potential causative agents.
With no definitive causal agent of EBA identified, the California Cattlemen's Association gave UCCE a grant from its Livestock Memorial Research Fund to develop educational outreach through a video on how to manage cattle to minimize the impact of the disease. Farm advisors and specialists with knowledge of the tick's feeding habitats and how the abortions developed in cattle used case studies with ranchers to develop management options that ranchers could use to lessen the impact of the disease on their business. Successful practices included pre-exposing sexually mature heifers to known tick areas prior to breeding, avoiding tick areas during the critical 2 to 6 months of pregnancy and shifting from spring to fall calving in the most northern regions of the state.
SVM researchers and the California Animal Health and Food Safety (CAHFS) laboratory system, using a large number of aborted calves, were able to develop methods to identify foothill abortion in aborted calves. This knowledge was extended to practicing veterinarians working with ranches throughout the state.
In 2002, a SVM laboratory developed a reliable challenge system for experimental transmission of EBA that was used to establish that the causative agent was antibiotic susceptible. This report was quickly followed with a positive identification of the agent causing foothill abortion, a bacteria belonging to a very unusual group of slime bacteria; then referred to simply as the agent of EBA, the bacterial pathogen has now been unofficially named Pajaroellobacter abortibovis.
Other breakthroughs followed quickly. The cultivation of the bacteria in immunodeficient mice gave new life to research efforts. A vaccine development phase was initiated with over $200,000 from the California Cattlemen Association's Livestock Memorial Research Fund and financial support from SVM and their collaborators at the University of Nevada, Reno. In 2009, a small group of heifers were protected against experimental infection after they were immunized several weeks prior to breeding with a candidate vaccine that was both live and infectious.
The success of a second and larger trial in 2010 prompted SVM researchers to pursue product licensing with the U.S. Department of Agriculture (USDA) Center for Veterinary Biologics. Vaccine efficacy experiments were conducted in accordance with USDA regulations. University-owned heifers were immunized before breeding and then administered an artificial challenge with virulent bacteria at the peak of fetal susceptibility (100 days gestation). Vaccine field trials that combined USDA-required field safety trials with field efficacy were then initiated at the UC Sierra Foothill Research and Extension Center on UC Davis Department of Animal Science heifers, on heifers at University of Nevada, Reno, and on producer-owned beef herds in California and Nevada. Over 1,600 heifers were enrolled in these trials in 2011 and 2012. Additional funding for such a massive effort was provided to the SVM by UC's Office of the President via a UC Proof of Concept Discovery Grant (grant ID no. 212263).
Although the results of these studies are currently being assembled, preliminary assessment of the experimental vaccine indicates excellent protection against foothill abortion has been successfully demonstrated. All the successes realized to date were a result of SVM collaborations across the UC spectrum with CAHFS's diagnostic laboratory at UC Davis, the UC Davis Department of Animal Science, UCCE, the Sierra Foothill Research and Extension Center, and also with researchers at the University of Nevada, Reno.
Historically, specialty vaccines created for use only in California were licensed through the California Department of Food and Agriculture (CDFA). Currently, CDFA does not process new specialty vaccines, requiring researchers to work with the USDA to get the foothill abortion vaccine licensed. USDA requirements are more stringent than CDFA's requirements, as food animal vaccines must comply with the federal Virus-Serum-Toxin Act requirements. SVM and USDA are charting new territory as they work to certify the safety and efficacy of the vaccine. The developers of the vaccine at SVM are in the process of establishing a USDA-required vaccine seed, determining if production can be scaled up to a commercial level and identifying viable options for commercial production of the vaccine.
As the commercialization efforts proceed, researchers are fine-tuning the vaccination regime to address concerns over the prolonged persistence of the vaccine bacteria and the potential impact on embryonic mortality in animals bred within weeks following vaccination. These studies are being conducted using a combination of UC and private producer replacement heifers. The vaccine dose is being adjusted downward, and the time from vaccination to breeding is being extended. The vaccine cannot be administered to pregnant cattle. Skin reactions following vaccination suggest that the live bacterial pathogen can persist for up to 2 months. On the positive side, this bacterial persistence induces a solid immunity that likely lasts through the next breeding cycle and possibly beyond. Studies are under way to begin to address length of immunity.
The fact that the vaccine is live and infectious poses several unique challenges. For example, the cryopreserved bacteria must be transported and stored in liquid nitrogen, and the cost of purchasing the vaccine could also be high because of the cost of manufacturing — the live vaccine must be cultured in an immunodeficient mouse. The California Cattlemen's Association is working to develop a regional distribution system for the vaccine, which could become available within a couple of years.
Work is being conducted to develop a recombinant vaccine through genomic research. In a recombinant vaccine, the genomic sequence of candidate bacterial genes must first be established. Next, the genes must be expressed as protein and then combined with adjuvant(s) to construct candidate vaccines. A recombinant vaccine would be far less sensitive to temperature and would not require immunodeficient mice in the manufacturing process, thereby making the finished product much more cost effective and practical for on-ranch use.
Subsequent findings by the SVM researchers have also improved the diagnostic procedures for identifying foothill abortions at the CAHFS diagnostic laboratory at UC Davis and have provided additional important information for UCCE to extend to ranchers to confirm abortions caused by the disease. Researchers are now actively pursuing validation of a diagnostic assay that may allow ranchers in the future to identify cows that have been exposed to the tick. Such an assay could be used to establish susceptibility of naïve replacement heifers to foothill abortion or confirm whether the disease is present on a ranch.
Decades of hard work by UC researchers and educators all across the system have allowed the pieces of this difficult disease puzzle to come together in assisting California's cattle ranchers.
References
Blanchard MT, Chen CI, Anderson M, et al. Serial passage of the etiologic agent of epizootic bovine abortion in immunodeficient mice. Vet Microbiol. 2010. 144(1–2):177-82. DOI: 10.1016/j.vetmic.2010.01.002 [CrossRef] [PubMed]
Bushnell R, Oliver M, Nader G, Norman B.. Foothill Abortion. 1991. Davis: Veterinary Medicine Extension, Cooperative Extension, School of Veterinary Medicine, University of California.
King DP, Chen C-I, Blanchard MT, et al. Molecular identification of a novel deltaproteobacterium as the etiologic agent of epizootic bovine abortion (foothill abortion). J Clin Microbiol. 2005. 43(2):604-9. DOI: 10.1128/JCM.43.2.604-609.2005 [CrossRef] [PubMed]
Stott JL, Blanchard MT, Anderson M, et al. Experimental transmission of epizootic bovine abortion (foothill abortion). Vet Microbiol. 2002. 88(2):161-73. DOI: 10.1016/S0378-1135(02)00109-8 [CrossRef]