All Issues
Forest nurseries face critical choices with the loss of methyl bromide fumigation
Publication Information
California Agriculture 67(3):153-161. https://doi.org/10.3733/ca.v067n03p153
Published online July 01, 2013
Abstract
Forest nurseries in the western United States have relied for decades on methyl bromide to control soilborne pests. Numerous studies have investigated alternative fumigants, alternative application methods and nonfumigant approaches for their ability to reduce soilborne pest populations and produce quality, disease-free seedlings. We review the recent studies and identify where research is needed to assist the industry's transition away from methyl bromide. For the immediate, foreseeable future, an integrated approach combining nonfumigant and fumigant methods will provide the best strategy. Nevertheless, the industry may need to transition completely to container production if fumigant regulations become more restrictive.
Full text
The forest nursery industry in the western United States produces tree seedlings that are primarily used for reforestation. Many states in the region require that forest lands that have been harvested or destroyed by fire, diseases or insects be replanted with seedlings. Oregon and Washington lead the western states in the number of seedlings that are replanted each year. In Washington, approximately 50 million seedlings were planted in 2011 (pers. comm., J. Trobaugh, Webster Nursery, Olympia, WA); similar numbers were planted in Oregon (OFRI 2008). Using average planting densities of 150 to 350 seedlings per acre (0.4 ha), we estimate that 143,000 to 333,000 acres (58,000 to 135,000 ha) of forest land were planted in each state during the 2011 planting season.
To meet demand for seedlings, nurseries in the western states of California, Idaho, Montana, Oregon and Washington produced approximately 200 million seedlings in 2011, over half of which were 1- or 2-year-old conifer species (industry survey, Weiland 2011, of 16 of the largest forest nurseries in those states). Approximately 75% of these seedlings (150 million) were sold as bareroot stock, with the remaining 25% sold as containerized stock. Bareroot seedlings, grown in the field and shipped without soil surrounding their roots, have historically been favored because they are generally larger in size than containerized seedlings, and can be produced in greater numbers, at lower cost.
Suburbs have encroached onto land adjacent to some forest seedling nurseries, which significantly restricts growers’ use of fumigants.
During the last decade, the industry suffered a series of nursery closures, particularly of state- and federally-funded operations. In June 2011, for example, California closed its last state nursery, in Magalia, due to state budget reductions (CalFire 2011). This followed the closing of its container seedling production facility in Davis in 2003. Some closures were due to the decreased demand for seedlings during the recent economic recession. However, much of the long-term reduction in demand has been driven by a downward trend in annual timber harvests since 1989 (Adams et al. 2006).
When seedling production is limited, demand can suddenly outstrip supply, as often occurs after catastrophic forest fires. Seedlings can sometimes be procured locally, but often they must be purchased from out-of-state (industry survey, Weiland 2011). Imported seedlings must meet phytosanitary certification requirements, but new weeds, pathogens and quarantine pests may be introduced accidentally.
Current methyl bromide use
Pest management is a significant issue for forest nurseries. For decades, the industry has relied on methyl bromide (MB) in combination with chloropirin (Pic) to manage soilborne insects, weeds and pathogens (Enebak 2007). The general practice in the Pacific Northwest has been to fumigate in fall with methyl bromide plus chloropicrin (67:33 at 350 pounds per acre), crop for 2 years and finish with a year of bare fallow (in which fields are not planted and kept weed free) before repeating the cycle (Weiland et al. 2011).
In the absence of soilborne pest control, nurseries can experience significant losses in seedling yield and quality. Some weeds, such as yellow nutsedge (Cyperus esculentus), are of particular concern because they are quarantine pests in Oregon and Washington. Many growers are also concerned about the introduction of non-native pathogens, especially after the discovery of Phytophthora ramorum, the causal agent of sudden oak death, in the ornamental nursery trade.
Nonfumigated plot shows disease and weed pressure, right foreground. Fumigated plot is in background.
Closeup of nonfumigated plot shows disease and weed pressure, top; fumigated plot shows healthy Douglas-fir seedlings, above.
Methyl bromide application in bareroot forest nurseries has continued under critical use exemptions (CUEs) and quarantine and preshipment exemptions (QPSs). Many nurseries initially used CUEs to continue methyl bromide application while alternative fumigants were evaluated. However, as the amount of methyl bromide available to U.S. forest nurseries under CUEs decreased from 192.5 tons in 2005 (UNEP 2010b) to 34.2 tons in 2012, some nurseries switched to QPSs (Enebak 2007). From 2008 to 2009, there was an almost 75% increase in QPS methyl bromide consumption in the United States, part of which was attributed to the switch from CUEs (UNEP 2010a).
Currently, almost all surveyed private bareroot nurseries in the Pacific Northwest continue to use methyl bromide, under QPS exemption, as the main method of soilborne pest control (industry survey, Weiland 2011). In contrast, federal nurseries have turned almost exclusively to dazomet (Basamid), because of federal pressure to use the least toxic materials and also because these nurseries are in the more-arid regions of the western United States, where spring fumigation can be carried out more easily. Private nurseries located west of the Cascades receive abundant rain in the winter and spring, which makes spring dazomet application infeasible due to seedling phytotoxicity (James 2002).
Of the 14 largest nurseries (including state, federal and private nurseries) in the western region that include some bareroot production, approximately 70% rely on methyl bromide for soilborne pest control (industry survey, Weiland 2011). In 1993, Smith and Fraedrich reported that 80% of the nurseries in the region relied on the fumigant; and in 1981, the figure was 90% (Landis and Campbell 1989). Regardless of the decrease in use, the pressure to further reduce methyl bromide for soil fumigation continues. Given the amount of attention that the QPS issue has received (UNEP 2010a, 2010b), growers should expect that the QPS exemptions will end in the near future.
Recent changes to fumigant application regulations and pesticide labels have significantly limited the use of methyl bromide and other fumigants in forest nurseries. In particular, buffer zone requirements affected nurseries near new suburban growth. Even with buffer zone reduction credits, the new restrictions implemented in 2012 will place restrictions on bareroot forest nursery production, and in all likelihood, growers will eventually lose methyl bromide as a pest management tool.
Fortunately, a large number of reviews and independent studies have addressed methyl bromide alternatives. This review will focus on what has been learned from research over the last decade or so, primarily from the western region.
Alternative fumigants
Most of the emphasis in the forest nursery industry has been placed on alternative fumigant chemistries as replacements for methyl bromide. As of April 2013, the Environmental Protection Agency (EPA 2013) listed five fumigant alternatives (and no nonfumigant alternatives) for the industry: dazomet, metam sodium (Vapam, Busan), chloropicrin (Pic), dimethyl disulfide (DMDS), and 1,3-dichloropropene (1,3-D), and two combinations of these: 1,3-D and chloropicrin (Telone, Pic-Clor 60) and metam sodium and chloropicrin. Each of the five EPA-listed alternative fumigants have been used with mixed results in nursery trials.
The EPA lists propargyl bromide and sodium azide as alternatives under development, but these are not yet registered for use in forest nurseries. Unfortunately, iodomethane (methyl iodide), an effective methyl bromide alternative, was pulled from the U.S. market in March 2012 by Arysta Life Sciences in response to poor sales due partly to its higher cost (Weiland et al. 2011) and partly to the number of environmental restrictions that were being implemented (EPA 2013).
Dazomet, metam sodium.
Early conifer nursery studies focused on methyl isothiocyanate (MITC)–producing agents (dazomet, metam sodium) and their efficacy (Tanaka et al. 1986). Rates varied from 250 to 350 pounds per acre for dazomet and 50 to 100 gallons per acre for metam sodium. These and later results (Littke et al. 2002) identified serious operational inconsistencies in chemical incorporation, water application and disease control efficacy. MITC agents require water activation to achieve efficacy, and this reaction is sensitive to temperature (must be above 50°F). This limits their use to summer and fall applications; severe phytotoxicity can result from incomplete chemical volatilization during spring.
Other research showed that tarping with high-density polyethylene (HDPE) plastic, virtually impermeable film (VIF) or totally impermeable film (TIF) increased weed and disease control. Currently, metam sodium remains a viable component in alternative fumigant mixes with chloropicrin; it is more easily incorporated uniformly as a liquid than the dazomet granular formulation.
Chloropicrin.
Chloropicrin formulated with methyl bromide (98:2, 67:33 or 50:50 MB:Pic) has been part of the operational fumigant standard for decades in most industrial forest nurseries. Chloropicrin is an effective soil disease control agent when used alone at 100 to 300 pounds per acre. However, broad-spectrum weed control is generally lacking.
In the southeastern United States, where lighter sandy soils prevail, 300 pounds per acre chloropicrin was comparable to methyl bromide with chloropicrin (MB:Pic) over three pine seedling crop rotations, provided an effective herbicide regime was used to control weeds (Cram et al. 2007; South et al. 1997).
Our experience in the western United States suggests that chloropicrin does not penetrate as well as methyl bromide into heavier soils, thus requiring more emphasis on proper soil preparation prior to fumigation. As a spring fumigant, chloropicrin lingers in the soil and increases the risk of phytotoxicity to newly transplanted seedlings. Chloropicrin regulations currently require concentrations above 20% in fumigant mixtures to comply with safety standards, and buffer limitations curtail the high doses of chloropicrin required for its stand-alone use as a fumigant.
Today, chloropicrin is used effectively as an alternative fumigant when paired with other fumigant agents — iodomethane:chloropicrin (50:50 at 350 pounds per acre), 1,3-D:chloropicrin (Telone C35 at 350 pounds per acre), and metam sodium:chloropicrin (50 gallons per acre:122 pounds per acre) — and used in combination with VIF or TIF tarps. A great deal of reliance is currently placed on chloropicrin as a component in fumigant mixtures. However, chloropicrin was listed as a toxic air contaminant by the California Department of Pesticide Regulation in 2010, which may indicate that more restrictive regulations are in store in California (CDPR 2011).
Dimethyl disulfide (DMDS).
The fumigant DMDS also holds promise if odor issues can be solved. A reduced-rate treatment of DMDS and chloropicrin (80:20 at 60 gallons per acre) was successful in controlling weeds and pathogens; however, its use resulted in worker and neighbor complaints; the distinctive odor was still strong in treated plots more than a month after application (Weiland et al. 2011).
1,3-dichloropropene (1,3-D).
This fumigant is used with chloropicrin in forest seedling nurseries to improve weed control. However, few published studies address its use in forest nurseries, and most research involving 1,3-D has been conducted only in the southeastern United States, where soil and environmental conditions differ greatly from those in the West (Enebak et al. 2011; Enebak et al. 2012).
Iodomethane.
Relatively few studies have been conducted with iodomethane. Unlike other fumigants, iodomethane behaves in a similar manner to methyl bromide and was the most likely replacement in all performance aspects before it was withdrawn from the market. When it was available in 2011, serious issues with its price (Weiland et al. 2011) and regulation (Washington State Department of Agriculture denied registration due to environmental concerns) limited its deployment.
Iodomethane with chloropicrin has been successful in soil disease and weed control, in both spring (80:20 at 275 pounds per acre) and fall applications (50:50 at 175 to 350 pounds per acre) (unpublished industry data and Weiland et al. 2011).
Effects on seedling quality.
Alternative fumigants have produced varying effects on final seedling density and quality when compared to the industry standard, methyl bromide plus chloropicrin. In seedbed trials, germinant density with alternative fumigants can equal that of methyl bromide or, as in the case of some dazomet trials, result in losses of up to one-third of the seed sown as the result of phytotoxicity from residual fumigant (Littke et al. 2002).
Stunting is also commonly observed following the use of MITC agents. Attempts to manage undersized seedlings with fertilization were not successful (industry data, unpublished), and reduced seedling colonization by mycorrhizal fungi does not appear to be a factor. Tanaka et al. (1986) showed that ectomycorrhizal colonization of Douglas-fir (Pseudotsuga menziesii) was not significantly different in standard- versus dazomet-fumigated and nonfumigated soils even when twice the normal rate of methyl bromide with chloropicrin was applied. For alternative fumigants to be fully implemented, research is needed to overcome these inconsistent results on seedling growth and quality.
Factors affecting fumigant efficacy.
Fumigant efficacy is negatively affected by the presence of root debris in the field. Root tissues are more resistant to fumigant penetration than bulk soil, so excessive amounts of residual debris may reduce overall fumigant efficacy. In addition, soil bulk density is critical; soils with higher bulk density reduce fumigant efficacy against soilborne pests (Weiland et al. 2011). Finally, additional research is needed to determine the critical threshold (concentration × time; CT) values for various pathogens to fumigant gases. Methyl bromide toxicity values suggest that control of Pythium and Phytophthora species is easier to attain than control of other pathogens such as Fusarium, Phomopsis and Rhizoctonia (Munnecke et al. 1978). Similar data is lacking for the alternative fumigants.
Alternative application methods
A number of fumigation methods have been developed that reduce the amount of fumigant applied and/or increase fumigant retention time in soil. Some of these methods are not easily used in forest nurseries. The application of fumigants through buried drip lines, for example, is incompatible with several nursery cultural practices, such as cultivating for weeds, seeding or transplanting operations, and root pruning and wrenching to produce compact root systems. All could destroy buried irrigation lines. However, water treatments to seal in dazomet (James et al. 2004), low-permeability plastic films, and reduced-rate fumigants (Weiland et al. 2011) are becoming commonplace. The methods described below can be incorporated into a pest management program to provide credits to reduce buffer zone sizes.
High soil moisture.
Cultural practices to prepare fields for fumigation have focused on tillage and ripping to remove soil pans that hinder fumigant diffusion. New best management practices (BMPs), as defined in the EPA reregistration eligibility decisions (REDs), require higher soil moisture content (> ?15% dry weight basis) at the time of application to retard fumigant efflux from the soil. Previously, operational soil moisture content at fumigation was kept low (2% to 10% dry weight basis) in combination with deep soil ripping to achieve maximum fumigant penetration. Gan et al. (1999) suggested that higher soil moisture differentially affects fumigant behavior, increasing the degradation of 1,3-D but not MITC. Similarly, Wang et al. (2006) concluded soil moisture plays a critical role in the conversion, distribution and efficacy of alternative fumigants (MITC agents, chloropicrin, 1,3-D). In general, higher water content increased the conversion of dazomet or metam sodium to MITC, but also limited the distribution of fumigants in soil. Future alternative fumigant treatment studies are needed under standard soil environmental conditions to evaluate the best management practices recommendations.
VIF and TIF.
Placing VIF and TIF over fumigated soil reduces fumigant emissions by more than 90% compared to HDPE, which only reduces emissions by 50% (Gao et al. 2011). The advantage of these films is that fumigants are retained in the soil longer, thereby increasing the amount of time that pathogens are exposed to toxic levels of the gas, which should increase disease control efficacy. One drawback, however, is that the waiting period for film removal should be longer than 1 week to allow the retained fumigant to degrade (Gao et al. 2011); otherwise, workers may be exposed to an emission surge when the film is cut. Recent experience with VIF and TIF combined with improvements in soil incorporation and sealing techniques warrants the review and retesting of soil fumigant treatments that were previously determined to be inadequate for forest nursery production under HDPE (e.g., early studies with lower rates of dazomet and metam sodium).
Application of virtually impermeable film (VIF) over a reduced rate of iodomethane plus chloropicrin. Glue (red strips) is released from the spray nozzle before the film unrolls (insert).
Reduced fumigant rates.
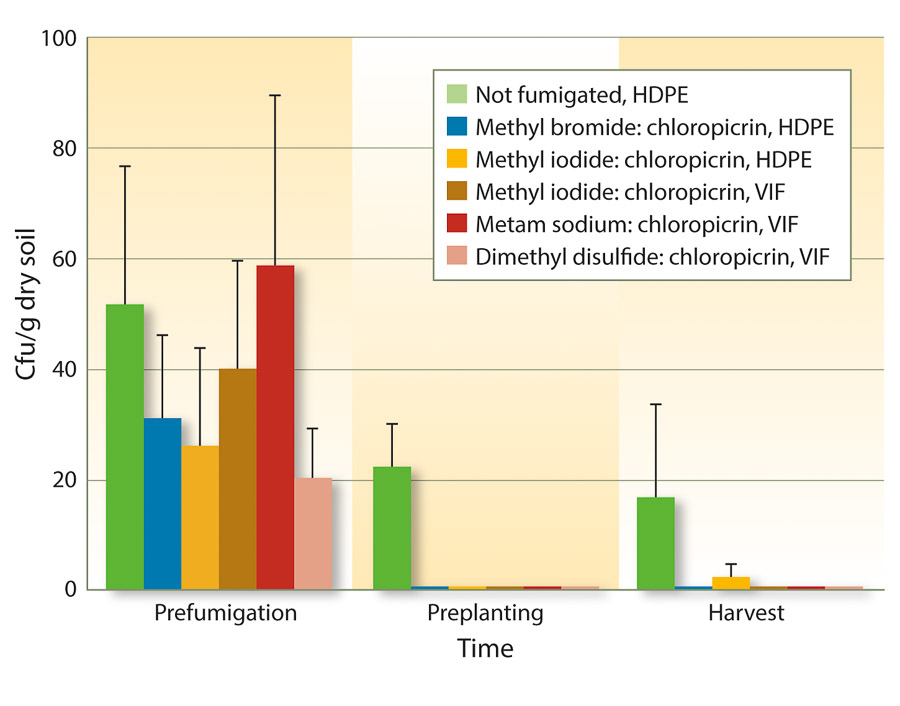
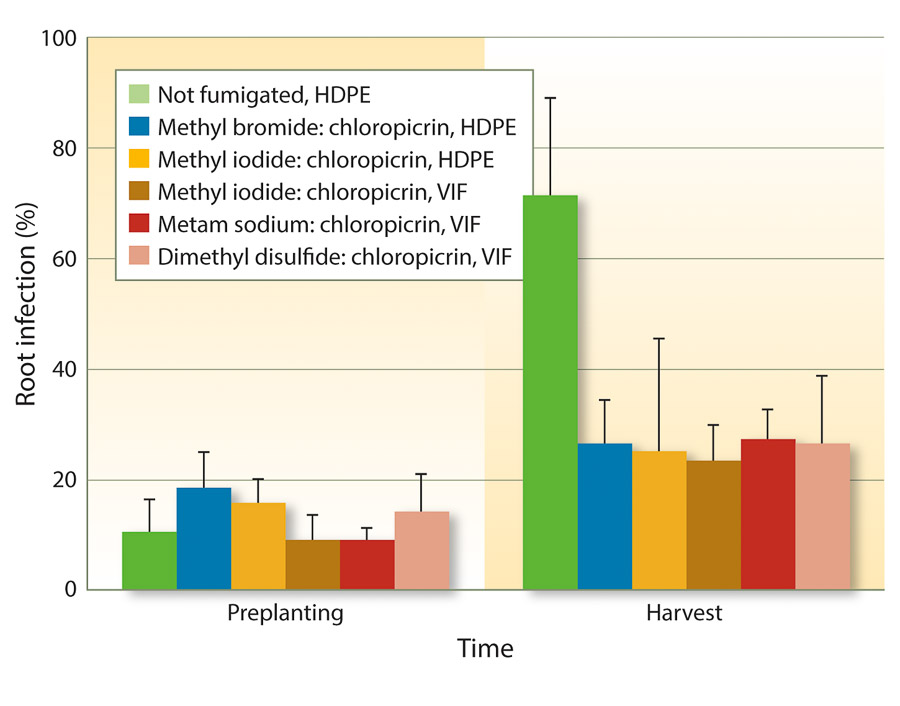
Because VIF and TIF retain soil fumigants for longer periods of time, fumigant rates may be reduced to achieve similar efficacy as full rates. One study from three forest nurseries in Oregon and Washington found that reduced rates of fumigants could be used under VIF for control of Fusarium and Pythium species (Weiland et al. 2011). Specifically, iodomethane:chloropicrin (50:50 at 244 pounds per acre), metam sodium:chloropicrin (50 gallons:122 pounds per acre), and DMDS:chloropicrin (80:20 at 60 gallons per acre) under VIF were as effective as methyl bromide:chloropicrin (67:33 at 350 pounds per acre) under HDPE. These results also held true for Cylindrocarpon species (figs. 1 and 2).
Fig. 1. Mean soil populations of Cylindrocarpon species (± SE) before fumigation in August 2008, 6 months after fumigation but just before planting in spring 2009, and at the end of the growing season in November 2009 in six fumigation treatments applied at three forest seedling nurseries (cfu = colony-forming units).
Fig. 2. Mean percentage of Douglas-fir seedling root infection (± SE) by Cylindrocarpon species before planting into fumigant treatments in spring 2009 and at the end of the growing season in November 2009 in six fumigation treatments applied at three forest nurseries.
Reduced-rate treatments are a potential option for nurseries with large buffer zones. To recover planting space, the center of a nursery field could be fumigated at the full rate, and then a reduced rate could be applied under TIF to the buffer zone of the full-rate fumigant. This second application would reduce the size of the required buffer zone at the field edge. The area treated with reduced-rate fumigant could be used for transplants, which do not require as stringent of a treatment as seedbeds.
Bed fumigation.
Another method that could reduce the amount of fumigant applied is using bed (or row) fumigation in place of flat (or whole-field) fumigation. It reduces the area to which fumigant is applied, but it's unknown whether the nonfumigated tire path between beds would serve as a reservoir for weeds and soilborne pathogens. If subsequent cultural operations, such as root wrenching and weeding, resulted in significant mixing of nonfumigated and fumigated soil, recontamination of the newly fumigated beds might occur more rapidly than if the entire field had been fumigated. One might suspect that the risk is relatively high; however, a previous study found that pathogen populations remained low in fumigated beds “despite the immediate proximity of unfumigated beds and the repeated movement of tractors and irrigation water across the plots” (Hansen et al. 1990).
It may be that if the nonfumigated region between beds can be maintained weed-free for several consecutive years, pathogen densities there would eventually drop, reducing the risk of recontamination over time. Also, soil preparations and nutrient additions might be made prior to fumigation to reduce postfumigation mixing of soils.
One final drawback to bed fumigation is the perception that there is a greater loss of fumigant around tarp edges. Fumigants might dissipate more rapidly through the nontarped, unfumigated regions between beds than from a flat fumigated field that is entirely covered by plastic. Furthermore, the amount of land left unfumigated in the tire tracks is also relatively small and would not contribute much to a buffer zone credit.
Although some nurseries in the Pacific Northwest use bed fumigation with dazomet, it is not widely used with other fumigants. The success of bed fumigation in other agricultural systems is promising, and this application method may yet prove amenable to tree seedling production.
Nonfumigant methods
Although some studies have demonstrated the potential for nonfumigant methods to control nursery pathogens and pests, these practices have not been studied in depth. Often, these studies report inconsistent seedling density and quality as well as a concomitant increase in the populations of potentially pathogenic microbes (e.g., Fusarium and Pythium species).
An increase in the populations of Fusarium or Pythium species does not necessarily mean that there will be an increase in disease incidence or severity. The populations can be pathogenic or nonpathogenic and may occur with other factors such as increased microbial diversity, better plant health and suppressed disease development (James and Dumroese 2007; Stewart et al. 2006). Nevertheless, methods to easily distinguish pathogenic from nonpathogenic populations are not currently available, and nursery managers must make disease management decisions without this information.
Because of these issues, the understanding and development of nonfumigant treatments lag behind those of alternative fumigant treatments and have limited the widespread adoption of nonfumigant methods by growers. To achieve adequate disease control, combinations of nonfumigant methods need to be investigated, possibly in rotation with fumigants or fungicides.
Bare fallow.
Bare fallow, maintaining a vegetation-free condition for a period of time, is a nonfumigant treatment, though it is not chemical-free, because it requires the use of herbicides to keep the ground bare. Weeds, weed residues, cover crops and green manures increase the organic residue in the soil, and pathogens such as Fusarium and Pythium species can survive in soil as facultative saprobes on these simple organic substrates when plant hosts are absent.
Hansen et al. (1990) found that grass or legume cover crops increased pathogen population densities over those in bare fallow plots throughout the crop cycle in nonfumigated conifer seedling beds (in forest nurseries). Similarly, a corn green manure crop resulted in high Fusarium levels (> 1,000 colony-forming units per gram) that persisted through a subsequent 2-year fallow period (James 2000). Fusarium species readily colonized soil organic matter, particularly roots of the previous conifer seedling crop, as well as the organic corn debris (James 2000).
Bare fallow in the season before planting can be effective in reducing pathogen populations by depleting the food base for facultatively saprobic pathogens (those that can survive on dead organic matter as well as cause disease on living plants). Additionally, bare fallow during the summer months may further reduce populations of pathogens, such as Pythium species, which thrive in moist conditions. In some cases, this reduction is enough to produce plant densities, seedling heights and stem diameters similar to those produced in fumigated plots (Hildebrand et al. 2004). In other studies, however, the reduction in pathogen populations has not been enough to reduce damage in comparison to fumigated areas (James 2001). Furthermore, bare fallow can leave some soils susceptible to wind erosion.
Organic amendments.
Organic amendments, which are used regularly in forest nurseries to improve soil physical and chemical properties, have been shown to stimulate bacteria, fungi and other soil organisms that can suppress soil pathogens. The effects vary among different amendments.
Aged sawdust (with delayed nitrogen application) benefited conifer seedlings over mature composts in USDA Forest Service nursery trials (Hildebrand et al. 2004). This was attributed to the sawdust's slow decomposition possibly favoring the growth of competitive soil saprobes to the detriment of soil pathogens that use simple organic substrates. Similarly, Barnard et al. (1997) found that materials with high carbon-to-nitrogen (C:N) ratios, such as composted pine bark, resulted in better disease suppression and seedling quality than materials with low carbon-to-nitrogen ratios.
Regardless, organic amendments do not achieve the level of disease suppression found with chemical fumigation (James 2001). Khadduri (2010) examined Douglas-fir seedlings planted into compost-amended soil that had either been spring fumigated with a methyl bromide:chloropicrin combination or left unfumigated. Although plots with biosolid and bark-based composts had the highest average number of packable seedlings relative to other compost amendments, seedlings raised in fumigated soil had better nutrient, pathology, morphology and packout measurements than seedlings grown in nonfumigated soil, regardless of compost treatment.
Brassicaceous plants (e.g., Brassica, Sinapsis and Limnanthes species) as a green manure cover crop may provide some biocontrol properties when they are incorporated into the soil. Upon decomposition, glucosinolates produced in the plant tissues convert to isothiocyanates, which can be toxic to soilborne pathogens including Pythium and nematode species (James et al. 2004; Zasada et al. 2012). However, results have been mixed.
In forest nursery studies, James et al. (2004) found a dramatic increase in Fusarium species populations and seedling mortality following incorporation of Brassica juncea. It appeared that insufficient toxicity levels in combination with increased organic matter resulted in an unintended favorable environment for pathogens. Glucosinolate degradation products, including isothiocyanates, can also be phytotoxic and are known to inhibit seed germination and seedling growth (Haramoto and Gallandt 2005). Given the sensitivity of conifer seedlings to MITC, the impact of brassicaceous cover crops on crop germination and stunting cannot be discounted and should be considered in future experiments involving brassicaceous species for pathogen control.
Biological control.
Most of the recent studies on the use of biological control agents in forest nurseries show they had little to no success in controlling root pathogens. Linderman et al. (2008), for example, tested 10 biological control agent formulations against damping-off caused by Fusarium oxysporum and Pythium irregulare in inoculated greenhouse-grown Douglas-fir seedlings. None was effective in reducing the incidence of damping-off. Similar observations were reported from other trials including field applications of biological control agents (Hildebrand et al. 2004; James et al. 2004), though there have been a few indications of success with biological control agents and mycorrhizae (Ocamb et al. 1997). Given the difficulty in achieving success, however, it is unlikely that biological control will play much of a role in nursery pest management without additional studies.
Abiotic environment modification.
Solarization and application of steam are considered impractical for forest nurseries in the West, especially those in the relatively cool climates of western Oregon and Washington. Solarization can be somewhat effective in those areas, but is limited by the number of sunny days (Hildebrand 1989). For example, Pinkerton et al. (2002) found that populations of Pythium, Phytophthora, Rhizoctonia and Cylindrocarpon, but not Fusarium, were reduced in solarization studies involving strawberries and red raspberries in Oregon and Washington.
Although solarization did not eliminate all pathogens and its efficacy diminished with increasing soil depth (due to a decrease in ambient soil temperature), it might prove useful prior to fumigation with reduced-rate fumigants or in combination with bare fallow. Similarly, steam injection has some promise but requires significant energy and time inputs to be effective (James et al. 2004) and, when soils are cool and moist, it can be impossible to bring soil temperatures up to target levels for an adequate duration.
The most effective abiotic treatment by far is water management. Strategic irrigation timing and frequency, along with treatments to keep the soil well drained, are critical for disease management (Dumroese and James 2005).
Containerized production.
Some growers have switched from direct sowing in nursery beds to sowing in small, containerized seedling plugs (e.g., miniplugs) or other containers (e.g., Cone-tainers or Styroblocks) in a greenhouse. In certain cases, hybrid production systems are used, in which seedlings are started in containers in a greenhouse and then transplanted into outdoor nursery beds. As long as some general sanitary precautions are taken (Dumroese and James 2005), containerized seedling production reduces some of the risks associated with soilborne pathogens and inclement weather and decreases the necessity for, or frequency of, soil fumigation.
As the forest nursery industry transitions away from methyl bromide, combining nonfumigant methods such as container production with fumigants will provide the best strategy for disease control. Above, container production in the Weyerhaeuser Rochester Greenhouse, Rochester, WA.
Very young, succulent seedlings are considered the most susceptible to infection by soilborne pathogens. In the field, seed beds are fumigated annually to reduce the risk of damping-off. Disease risk is further reduced by using raised beds and warm planting temperatures to promote rapid germination and seedling establishment, and by preventing excessive succulence with lower levels of nitrogen fertilization. Containerized systems reduce disease risk by starting seeds in a clean, protected environment such as a greenhouse. Growers must use sterile containers and soilless media, clean irrigation water, and disinfested seeds. Once the seedlings have reached an appropriate size, they can be sold directly as container stock or transplanted into field beds to produce larger bareroot plants, although transplant beds require periodic fumigation. In a controlled, greenhouse environment, the seeds may be sown at an earlier date than in the field. If timed correctly, this process can remove 1 year from the production cycle (Riley and Steinfeld 2005).
In the West, approximately 25% of the forest nursery seedlings are produced in containers. The infrastructure needed to produce the additional 150 million seedlings in containerized systems would require a large expenditure of capital. Annual expenditures for heating, additional labor, and supplies (containers and planting media) would also be incurred and shipping costs would rise, as containerized seedlings are more expensive to ship due to their bulk and weight. As a result of these costs, containerized seedlings are consistently more expensive than bareroot stock. The price of similarly-sized containerized seedlings can be approximately double those of bareroot seedlings, while those from hybrid production systems (e.g., plug plus 1 year in transplant bed) can be up to 20% more expensive.
Research needs
As the forest nursery industry transitions away from methyl bromide, more knowledge is needed about a number of critical issues.
Pathogen identification.
For the most part, methyl bromide's success limited research about the species identity of soilborne pathogens that commonly affect tree seedling production; as long as it was applied correctly, there was little need to identify the pathogens to achieve adequate control. In contrast, effective nonfumigant practices generally rely on accurate species identification and knowledge of pathogen biology.
Although it is generally known which genera cause disease (e.g., Cylindrocarpon, Fusarium and Pythium), little is known about which particular strains or species are pathogenic. Accordingly, caution should be used when interpreting soil population values as a criterion that fumigation needs to occur, or as a measure of treatment efficacy. Studies have demonstrated that high populations of Fusarium or Pythium do not always correlate with seedling damage and mortality (Hansen et al. 1990; Hildebrand et al. 2004), because the populations may include nonpathogenic isolates. What is more important to know is the proportion of pathogenic to nonpathogenic isolates within the soil population. The greater the number of pathogenic isolates in proportion to the nonpathogenic isolates, the greater the risk for disease.
Progress is being made on pathogen identification. Much of the groundwork was accomplished by R. L. James and colleagues, who identified pathogenic species of Fusarium and Pythium (e.g., James 2002). More recently, a number of new Fusarium and Pythium species have been described from forest nursery soils (Weiland 2011; Weiland et al. 2011), and research has identified eight Pythium species that are virulent pathogens of Douglas-fir seedlings (Weiland et al. 2013).
Tools have also been developed that distinguish between pathogenic and nonpathogenic isolates. Stewart et al. (2006), for example, found genetic markers that differentiated nonpathogenic isolates of Fusarium oxysporum from pathogenic isolates of F. commune. In addition, newer technologies (e.g., multi-pathogen detection arrays) are becoming available that allow for rapid screening of soil samples for multiple plant pathogens. Future research should continue to focus on the identification and differentiation of pathogenic and nonpathogenic species as well as the development of technologies to quickly evaluate soil populations for their potential to cause disease.
Pathogen monitoring.
Pathogen populations may shift in response to new fumigant chemistries and rates, or in response to changes in disease management methods. For example, reduced-rate formulations may select for pathogens that can survive lower doses of fumigant, thus increasing the risk for developing pesticide resistance. Similarly, new pathogens, or previously minor pathogens that were controlled by methyl bromide, may become problematic if they are less sensitive to other fumigant chemistries. Many alternative disease control methods are new to the forest nursery industry and have been tested under a relatively narrow range of environmental conditions; their long-term impacts on pathogen populations remain unknown. Periodic monitoring for newly emergent pathogens should help the industry avoid unexpected losses.
There is little information about the movement of soilborne pathogens from nursery to nursery on infested planting stock. Hansen et al. (1979) found evidence that this has occurred in the Pacific Northwest forest nursery industry, but additional research is needed to fully evaluate the risks. This issue is particularly important given the experience of the ornamental nursery industry with Phytophthora ramorum and may become even more pressing if the movement of planting stock among forest nurseries increases in response to nursery closures. In the meantime, nurseries that receive stock should carefully inspect for evidence of disease.
Spring fumigants.
Many private nurseries in the region rely on fall fumigation to ensure that enough land is available to meet spring production demands. However, customer orders continue to arrive throughout the winter months and additional land may need to be fumigated in the spring. There is little evidence about whether alternative fumigants perform as well as methyl bromide under spring environmental conditions. Phytotoxic effects may be observed if the fumigants linger in cool, moist soil for long periods of time; dazomet, for example, can damage young seedlings when applied in spring immediately before planting (James 2002).
Soil temperatures higher than 50°F (10°C) are also needed before dazomet and other MITC agents become active. These soil temperatures are often not reached until mid-April at some nurseries, and fumigation at that time would push back sowing to mid- or late May, resulting in a shorter growing season and smaller seedlings. Several of the new fumigant chemistries appear to be adequate for fall fumigation; however, research is critically needed to evaluate their appropriateness for spring fumigation.
Precision application.
Additional research is warranted into precision fumigation, which involves the modification of equipment to more precisely deliver fumigants at the desired rate and injection depth to specific locations within a field (Sances et al. 2008; Upadhyaya et al. 2009). Precision fumigation uses GPS technology, coupled to a shank-type applicator, to determine the correct location in the field to begin and end fumigation. Although these technologies are at the beginning stages of development and testing, they could eventually play an important role in reducing the amount of applied fumigant and may be particularly valuable in testing the feasibility of bed fumigation for the forest nursery industry. One estimate from an early prototype indicated that fumigation rates could be reduced by approximately 50% (Upadhyaya et al. 2009).
Integrated pest management.
Perhaps the most important research need is the continued development of integrated pest management (IPM). Used alone, many alternative methods of soilborne pest control may never be as effective as methyl bromide. However, if they can be coordinated into a cohesive integrated pest management program, successful pest control might be achieved. Weiland et al. (2011), for example, found promising results in nurseries that used bare fallow in combination with reduced-rate fumigants under VIF.
To be sure, integrating multiple approaches is more complicated than traditional methyl bromide fumigation, but given the political and public emphasis on environmental health and sustainability, it is likely that government agencies will continue to strictly regulate fumigant application. Integrating nontoxic methods to limit pathogen establishment and population growth (e.g., bare fallow, proper irrigation practices and drainage, and recycled water treatment) and practices that reduce fumigant use without compromising disease control (e.g., low-permeability plastic films and reduced-rate fumigant formulations) appears to be the best strategy at this time. Furthermore, as pathogen identification technologies improve, it will become possible for nursery managers to tailor a program to target soilborne pests that predominate at their location.
Research on the risks associated with the absence of any disease control treatment over an extended period of time (i.e., > 2 years) would enhance the usefulness of information from integrated pest management studies. Data from long-term, nontreated field plots could be used to determine the pathogen-carrying capacity of nursery soils, examine the likelihood of developing disease-suppressive soils, and establish whether there are natural fluctuations in pathogen populations that can be exploited to enhance disease control.
Progress and outlook
In 1994, Linderman et al. reported research priorities for the forest and ornamental nursery industries as identified from the 1993 USDA workshop on methyl bromide alternatives. The short-term priorities were to develop 1) new chemicals (including fumigants), chemical application technologies and optimal application rates; 2) integrated pest management systems integrating existing chemical, cultural, physical and biological control practices; and 3) new crop production systems. For the most part, many nurseries are still not using alternative fumigants because of cost and the perceived inconsistencies in control. Growers are also wary about the continued availability of alternative fumigants given frequent changes in environmental regulations and fumigant application rules. Although not established yet, optimal application rates for alternative fumigant chemistries are being developed as new technologies (precision delivery, low-permeability plastic films) are combined with reduced fumigant rates in field trial evaluations. However, until all fumigant chemistries are taken off the market, there will likely be little progress in developing an integrated pest management system that does not include some component of soil fumigation for bareroot seedling production. Forest nurseries in Canada, and a few in the United States, have transitioned to containerized production successfully. Nevertheless, the current demand for seedlings in the United States cannot be met by the existing infrastructure for container seedling production.
Long-term priorities identified by Linderman et al. (1994) included pest-resistant hosts, safer chemicals that target specific pests, biological control, soil solarization, and pasteurization or other heat treatment methods. Interest was also expressed in the detection of pest populations and forecasting pest damage. Unfortunately, there has been little progress toward these goals. To our knowledge, little to no research has been conducted regarding genetic host resistance against soilborne pests; most resistance-screening programs have targeted host-specific pathogens such as the foliar and stem rusts of pine. Heat treatment and biological control methods have been tested and are considered too expensive, impractical, or of limited efficacy at this stage of development (Hildebrand 1989; James et al. 2004; Pinkerton et al. 2002). However, new pesticide chemistries are continuously being tested for their efficacy against specific pests (Zasada et al. 2012), and progress is being made in pathogen identification and detection (Weiland 2011; Weiland et al. 2013).
In many ways, the forest nursery industry is still in the same position as almost 20 years ago; many growers still rely on methyl bromide, and the fumigant is expected to be eliminated in the near future. The range of environments, crops and pest species makes it nearly impossible to develop a single, nationwide solution for forest nursery pest management that will replace methyl bromide. In the short term, many will opt to continue using methyl bromide until it is completely removed from the market. Once it has been eliminated, growers will likely switch to alternative fumigant chemistries, which offer broader pesticidal activity and better consistency in control than currently available nonchemical disease control strategies. If fumigation is ever completely eliminated as a pest control strategy, one potential solution would be container production. A second alternative would be to attempt bareroot production in the absence of fumigation by using the best integrated pest management methods available, which would likely include a concomitant increase in herbicide and fungicide use. However, it is generally assumed that this strategy would be largely ineffective and result in significantly fewer and smaller seedlings of lesser quality.
The current challenge is to integrate newer, promising pest control measures (alternative fumigant chemistries and application methods) with existing nursery practices (field preparation, soil moisture and fertility management, seedling densities) and nonfumigant disease control measures (bare fallow, fungicide application) to achieve a successful pest management program. Each of these separately can provide a certain amount of pest control. However, coming up with the optimal combination of strategies that will work under the widest range of conditions and locations will be difficult. Continued support from the forest nursery industry, as well as state and federal agencies and research universities is critical for conducting the necessary research and will ensure that healthy seedlings will continue to be produced for restocking U.S. forest lands.