All Issues
Biocontrol program targets Asian citrus psyllid in California's urban areas
Publication Information
California Agriculture 71(3):169-177. https://doi.org/10.3733/ca.2017a0027
Published online September 13, 2017
PDF | Citation | Permissions
Abstract
In California, Asian citrus psyllid vectors the bacterium Candidatus Liberibacter asiaticus, which causes the lethal citrus disease huanglongbing. The top priority for California's citrus industry has been to diminish the rate of bacterium spread by reducing Asian citrus psyllid populations in urban areas, where this pest primarily resides. Attempts at eradicating and containing the psyllid with insecticides were unsuccessful. An alternative approach has been a classical biological control program using two parasitoids from Pakistan, Tamarixia radiata and Diaphorencyrtus aligarhensis, which attack the psyllid nymphs. T. radiata has established widely and, in combination with generalist predators, natural enemies are providing substantial control of psyllids in urban areas.
Full text
Asian citrus psyllid (ACP), Diaphorina citri Kuwayama (Hemiptera: Liviidae), has emerged as the most important exotic insect pest of citrus in California. Damage is two-fold. First, psyllids cause direct injury to citrus through feeding on phloem juice in immature foliage, deforming the leaves (Halbert and Manjunath 2004); and second, and more importantly, they vector the bacterium Candidatus Liberibacter asiaticus (CLas), which causes the lethal and untreatable citrus disease, huanglongbing (HLB), also called citrus greening disease.
Characteristic symptoms associated with CLas infection are reduced vigor, foliar discoloration and dieback, misshapen fruit with bitter juice and malformed seeds, premature fruit drop, overall yield reductions and, ultimately, tree death (Gottwald 2010). Though symptoms may not appear for several years, CLas-infected plants are bacteria reservoirs from which ACP acquires and spreads the HLB-causing pathogen. CLas spread is exacerbated prior to detection if ACP populations are high and not managed. While there are some differences in susceptibility across citrus varieties, virtually all commercially available varieties are vulnerable to CLas infection.
HLB is the most important vector-borne disease threat to the citrus industry in the United States. ACP and CLas were first found in the United States in Florida in 1998 and 2005, respectively (Grafton-Cardwell et al. 2013). Since then, the ACP-CLas pathosystem has been detected in six U.S. states, and ACP establishment has been confirmed in 10 U.S. states.
Asian citrus psyllids spread huanglongbing, a lethal citrus disease that poses a serious threat to the U.S. citrus industry. Psyllid populations are now established in urban areas of Southern California, where a classical biological control program with parasitoids from Pakistan is attempting to reduce psyllid numbers and migration into commercial citrus production areas.
The emergence of HLB in Florida citrus orchards has had significant economic impacts. Approximately 75% of all citrus trees grown in Florida are infected with CLas. Consequently, production costs have increased by 33% because of increased ACP-CLas management, and productive acreage has declined by 44% (from a high of 815,100 acres in 1996 to 459,100 acres in 2015) (USDA NASS 2016a). Financial losses credited to ACP-CLas have been estimated at $3.6 billion, including over 8,000 jobs lost (Spreen et al. 2014).
In California, ACP was first detected in August 2008 in San Diego County. The pest is found primarily in urban citrus grown in Southern California (Kistner, Amrich et al. 2016). The first CLas-infected tree was discovered in a private residential garden in Hacienda Heights in Los Angeles County in December 2012 (Kumagai et al. 2013). As of May 2017, about 60 trees infected with CLas in Los Angeles and Orange counties have been confirmed by the California Department of Food and Agriculture (CDFA).
ACP-CLas poses a serious threat to California's $3 billion-a-year citrus industry, which employs over 26,000 people (Richards et al. 2014). California is the number one producer of fresh-market citrus in the United States, supplying more than 85% of the oranges, mandarins and tangerines and over 90% of the lemons (USDA NASS 2016b). There is zero industry tolerance for misshapen and bitter fruit in California's fresh-market citrus crop. California growers are already faced with escalating ACP-HLB management costs, and economic forecasts indicate that expenses associated with ACP-CLas may rise to $220 million a year in the next 5 years (Bennet 2016).
CLas spreads rapidly through ACP populations and citrus groves (Hall et al. 2013). Citrus orchards may become economically unfeasible 2 to 5 years following infection of the first tree (Bassanezi and Bassanezi 2008). The most important contributing factors to rapid spread of CLas by ACP are (A) the high dispersal potential of adult psyllids, which is facilitated by their good flight capabilities and small size, enabling them to be blown long distances by wind and go undetected on shipped plants and (B) rapid psyllid population growth, which results from short generation times and the high fecundity of females (Lewis-Rosenblum et al. 2015). Additionally, uninfected psyllids are more attracted to CLas-infected trees than to uninfected trees, which may further facilitate rapid pathogen spread (Mann et al. 2012). Thus, suppressing ACP populations is important for reducing CLas movement, which in turn maximizes orchard longevity and productivity.
Growing concerns about ACP
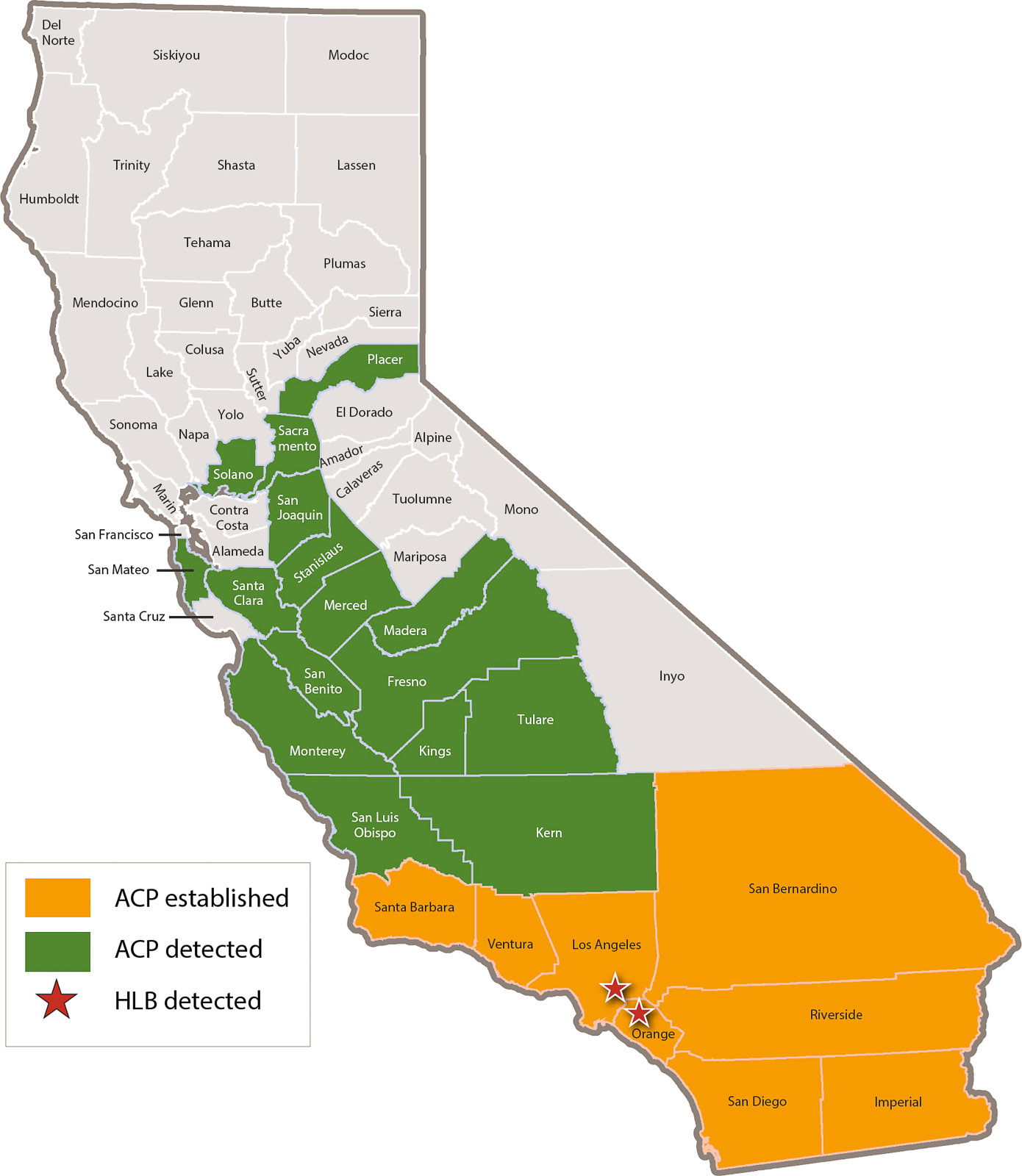
As of 2017, ACP populations have been found in 24 counties throughout California (CDFA 2017a), including the major commercial citrus production area in the San Joaquin Valley, which accounts for ∼ 77% of California's citrus industry (fig. 1) (USDA NASS 2016b). Fortunately, the commercial production areas have not yet suffered from the widespread establishment of the ACP-CLas complex. The distribution of CLas has remained largely restricted to Hacienda Heights, San Gabriel and Cerritos in Los Angeles County and Anaheim in Orange County (fig. 1).
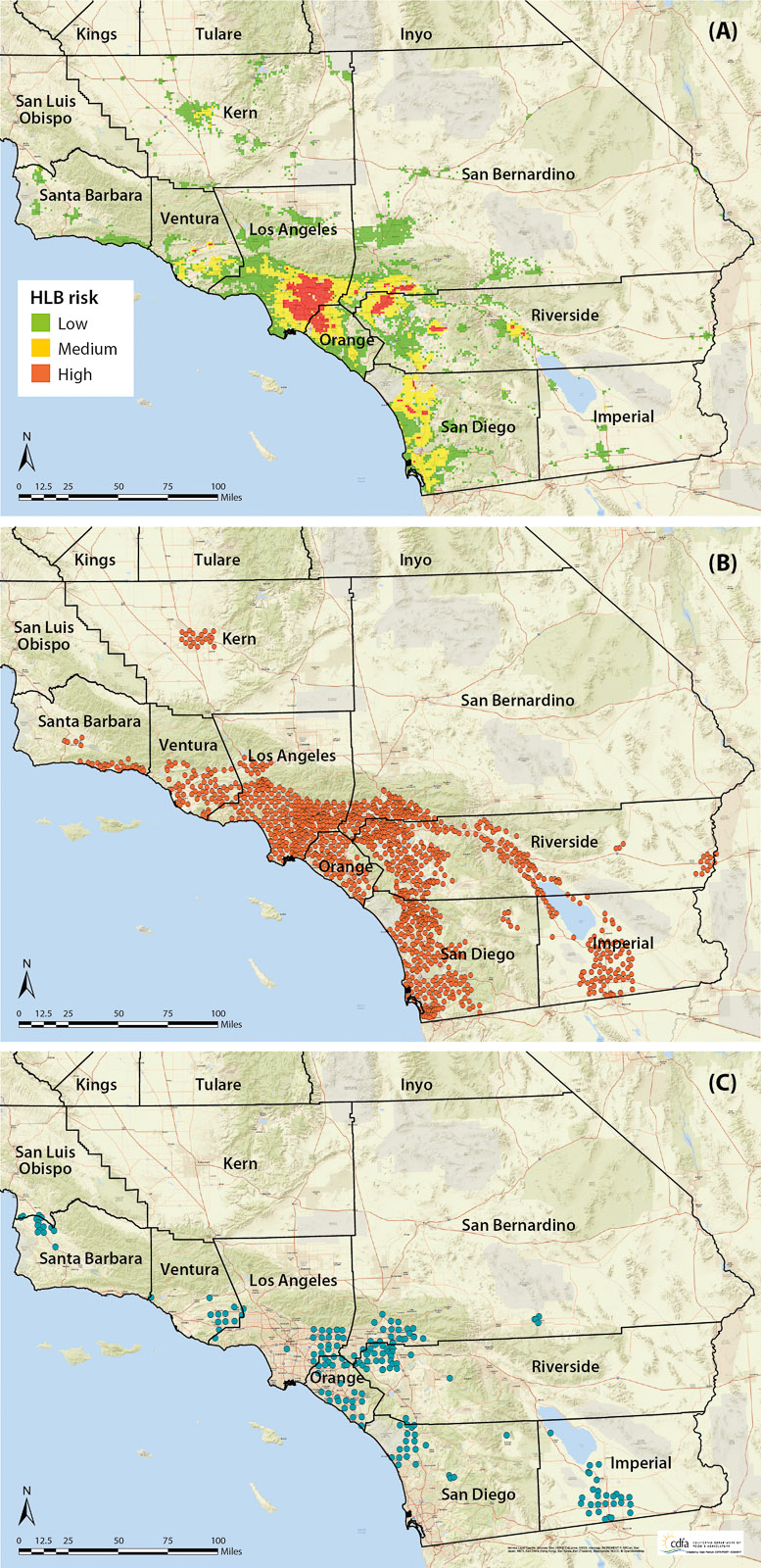
A heat risk map for HLB in Southern California has been developed by the U.S. Department of Agriculture (USDA), and this probabilistic map is used to direct ACP natural enemy releases (see below for details on ACP parasitoids imported from Pakistan) in urban areas in accordance with perceived likelihood of the occurrence of CLas-infected trees (fig. 2A). As ACP continues to spread, there is an increased risk of ACP-CLas establishing in major citrus growing areas of California.
Fig. 2. (A) Huanglongbing risk grids generated by USDA researchers in 2016. Southern California release locations through 2016 of the psyllid parasitoids (B) Tamarixia radiata and (C) Diaphorencyrtus aligarhensis.
In an attempt to eradicate ACP populations in residential areas, the CDFA ran pesticide application and monitoring programs during the initial stages of the ACP invasion into Southern California (Hoddle 2012). Pesticide applications consisted of fast-acting cyfluthrin foliar sprays followed with slower-acting and persistent systemic imidacloprid soil drenches. While the CDFA spray program was moderately successful in killing ACP on infested backyard citrus, it was prohibitively expensive, at ∼ $200 million (approximately $100 per property), and it was estimated that less than 10% of residential citrus at risk of ACP infestation was treated. Consequently, the ACP urban spray program was largely terminated in 2012 after 3 years (Hoddle and Pandey 2014).
Following the first detection in San Diego County, ACP rapidly spread throughout Southern California. Established populations also have now been recorded in the Central Coast (2009), Central Valley (2012) and the greater San Francisco Bay Area (2014) (fig. 1). In response to this spread, a CDFA-appointed ACP quarantine first implemented in September 2008 in San Diego was initially restricted to 20 square miles (52 square kilometers) (Victoria Hornbaker, CDFA, personal communication); it has now increased and spans 24 counties encompassing over 62,000 square miles (160,000 square kilometers) (CDFA 2017a). The quarantine aims to reduce ACP-CLas spread by regulating the movement of citrus and closely related species (CDFA 2017b).
Backyard citrus represents a large portion of total citrus acreage in California. In Los Angeles County alone, it has been estimated that over 1.2 million residences may have at least one backyard citrus plant (Hoddle 2012). Residential areas are important reservoirs for ACP and CLas in California, and there is a high risk of infected vectors moving from these areas into commercial groves (Gottwald 2010).
Asian citrus psyllid life stages: (A) adult psyllid feeding on young tissue, (B, C) gravid adult females and eggs on citrus flush and (D) nymphs producing white honeydew secretions that are harvested by Argentine ants.
In Florida, abandoned citrus groves have been identified as significant reservoirs for ACP and CLas, with infected psyllids dispersing to commercial groves in spring and summer (Lewis-Rosenblum et al. 2015). Most commercial citrus production areas in California are naturally arid, so abandoned groves tend not to persist; however, abandoned citrus in moister coastal areas could become reservoirs. Neglected citrus groves may become more common across the state if CLas becomes more widely established and increased management costs make production unprofitable.
Huanglongbing symptoms, photographed in Florida: (A) irregular blotchy yellowing or mottling of leaves and (B) reduced plant vigor and foliar dieback in CLas-infected citrus trees in advanced stages of decline. Researchers estimate that the Florida citrus industry has lost $3.6 billion, including over 8,000 jobs, as a result of HLB (Spreen et al. 2014).
Native rutaceous plants (i.e., citrus relatives) are widely distributed in California wilderness areas, especially in strategically significant locations near commercial production areas in the San Joaquin and Coachella valleys and Imperial and San Diego counties (Calflora 2017). It is unknown if these wilderness areas harbor ACP-CLas populations or if they could act as potential spillover hot spots for ACP-CLas spread, as migrating ACP could introduce CLas into commercial citrus production areas (Grafton-Cardwell et al. 2013). In the absence of insecticide management, biological control of ACP may be the only feasible population suppression tool in areas (i.e., urban environments, wilderness areas, and organic or abandoned citrus orchards) not under active management.
Natural enemy prospecting program
Initial surveys of ACP populations in urban-grown citrus, and the subsequent rearing and dissection of recovered nymphs, revealed a lack of specialist natural enemies (e.g., parasitoids), which may be the reason ACP populations spread rapidly in Southern California (Hoddle 2004, 2012). This apparent lack of specialist natural enemies, coupled with the cessation of CDFA's urban spray programs for ACP control, prompted a search for effective biological control agents to suppress ACP population growth.
Beginning in September 2010, researchers at UC Riverside in collaboration with CDFA and USDA scientists initiated development of a classical biological control program targeting ACP populations in urban Southern California. Diaphorina citri has a native range that includes the Indian subcontinent (Beattie et al. 2009). Natural enemy prospecting was conducted in the Punjab province of Pakistan, because climate matching indicated that this area has a ∼ 70% climate match with the major citrus production areas in California's Central Valley (Hoddle and Pandey 2014). Biocontrol theory suggests that climate matching is important for improving the likelihood that a natural enemy will establish in the intended introduced range; preadaptation to the prevailing climate eases at least one establishment barrier (Van Driesche et al. 2008).
Hussain and Nath (1927) stated in their treatise on D. citri that the diversity of parasitoid species associated with ACP nymphs in Punjab was high, with possibly nine species being recorded attacking immature stages. Their work, however, resulted in the description of just one parasitoid species, Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae). The identities of the other species were not known when California's ACP biocontrol program commenced.
Foreign exploration efforts by UC Riverside scientists in Punjab were conducted in collaboration with researchers in the Department of Entomology at the University of Agriculture, Faisalabad. This excellent cooperative arrangement was essential for the successful collection of ACP natural enemies from citrus production areas around Sargodha, Toba Tek Singh, Faisalabad and Gujranwala.
UC researchers collaborated with researchers at the University of Agriculture, Faisalabad, in Punjab, Pakistan, to collect natural enemies of ACP. Left, fieldwork in some areas involved assistance from citrus farmers. Right, at the university citrus research orchard, Christina Hoddle (left) processed ACP-infested citrus cuttings before taking them to a lab to rear the parasitoids; here, she is working with students Shouket Zaman Khan (back center) and Saif ur Rehman (right front).
Safeguards to minimize risks
Potential natural enemy species from Punjab, Pakistan, were imported under a USDA APHIS permit into quarantine at UC Riverside from April 2011 to April 2013. These collections resulted in the rearing of 3,675 parasitoids from which 13 ACP-associated parasitoid species were identified (Hoddle et al. 2014). Two species were particularly highly represented: 55% of collected parasitoids were identified as T. radiata and 28% were Diaphorencyrtus aligarhensis (Shafee, Alam and Argarwal) (Hymenoptera: Encyrtidae) (Hoddle 2012).
The 11 other parasitoid species included three leaf miner parasitoids, two egg parasitoids, one scale parasitoid and five obligate hyperparasitoids of T. radiata and D. aligarhensis; these additional 11 parasitoid species do not attack ACP (Bistline-East and Hoddle 2014, 2016; Hoddle et al. 2014). Hussain and Nath's (1927) estimate of nine natural enemy species associated with ACP nymphs in Punjab was concluded to be incorrect; they had perhaps inadvertently assumed that parasitoids attacking other cryptic citrus pests (e.g., cicadellid eggs) on ACP-infested foliage were natural enemies of ACP (Hoddle et al. 2014).
Host range and host specificity studies concluded that the two primary ACP parasitoids, T. radiata and D. aligarhensis, likely posed no undue risk to nontarget psyllid species in California (Bistline-East and Hoddle 2014; Hoddle and Pandey 2014). These findings resulted in USDA APHIS issuing permits authorizing the release of T. radiata and D. aligarhensis for classical biological control of ACP in California.
California's ACP biocontrol program
T. radiata is a host-specific ectoparasitoid of fourth and fifth instar ACP nymphs. It was the first natural enemy species released in California, in December 2011. Female T. radiata kill ACP nymphs through a combination of parasitism and host feeding, which provides nutrients for parasitoid egg development. It has been estimated that a single female can kill up to 500 nymphs in her lifetime (Chien et al. 1995).
Adult Tamarixia radiata (A) male and (B) female. The latter kill Asian citrus psyllid nymphs through a combination of parasitism and host feeding.
Over the last 5 years, more than 5 million T. radiata have been mass-reared and released in Southern California in a collaborative effort by the CDFA, UC Riverside, USDA and the California citrus industry. Tamarixia radiata releases are made using a 1-square mile (2.6-square-kilometer) grid that spans more than 45,000 square miles (116,500 square kilometers) across nine counties in Southern California (fig. 2B). Releases have resulted in widespread establishment of T. radiata. Approximately 95% of surveyed release and nonrelease sites (n = 100) distributed in six counties had T. radiata activity, and detections indicated that the parasitoid had migrated into nonrelease locations (n = 28), with some finds at least 8 miles (∼ 13 kilometers) from the nearest release site (Hoddle et al. 2016). Molecular testing of field-recovered T. radiata indicated that they had genetic signatures unique to the Pakistan populations released in Southern California (Dr. Paul Rugman-Jones, UC Riverside, personal communication).
Postrelease monitoring in California indicates that the average parasitism of ACP nymphs by T. radiata is ∼ 20% (range is 13% to 63%), but it can vary greatly across study sites over time (Kistner, Amrich et al. 2016). Moreover, the combination of T. radiata and increased attacks by native predators, such as syrphid fly larvae, which have started using ACP nymphs for food, are having a substantial impact, reducing urban ACP populations by more than 90% at some locations at certain times of year. As of this writing (spring 2017), T. radiata releases are largely restricted to urban-grown citrus; releases are not made in commercial citrus production areas that are under area-wide management, where insecticide applications are coordinated over large areas and spray residues cause substantial mortality of T. radiata (Hall and Nguyen 2010).
Tamarixia radiata developmental biology: (A) female laying an egg underneath a psyllid nymph, (B) parasitoid egg (arrow) attached to its host, (C, D) T. radiata nymph feeding externally on its host, (E) developing T. radiata pupae removed from ACP mummies and (F) an adult T. radiata that has emerged from the anterior region (circular exit hole) of the ACP mummy. T. radiata has established widely in Southern California since releases began in late 2011.
In Florida, T. radiata imported from Taiwan and Vietnam have established widely (Hoy and Nguyen 2001). But their impact on ACP is not high, especially in comparison to coccinellid predator species, which appear to be significantly regulating ACP populations (Michaud 2004). The reasons for the putative poor performance of T. radiata in Florida are varied and may include the parasitoid's high sensitivity to pesticide residues in commercial citrus groves, low levels of genetic variation in released parasitoids that may have reduced their fitness and subsequent efficacy, and interference by ants tending honeydew-producing ACP nymphs (Navarrete et al. 2013). Studies in Florida have shown coccinellid predation of ACP nymphs already parasitized by T. radiata can result in over 95% mortality of developing T. radiata. This is an additional factor potentially contributing to low parasitism rates observed in Florida (Qureshi and Stansly 2009).
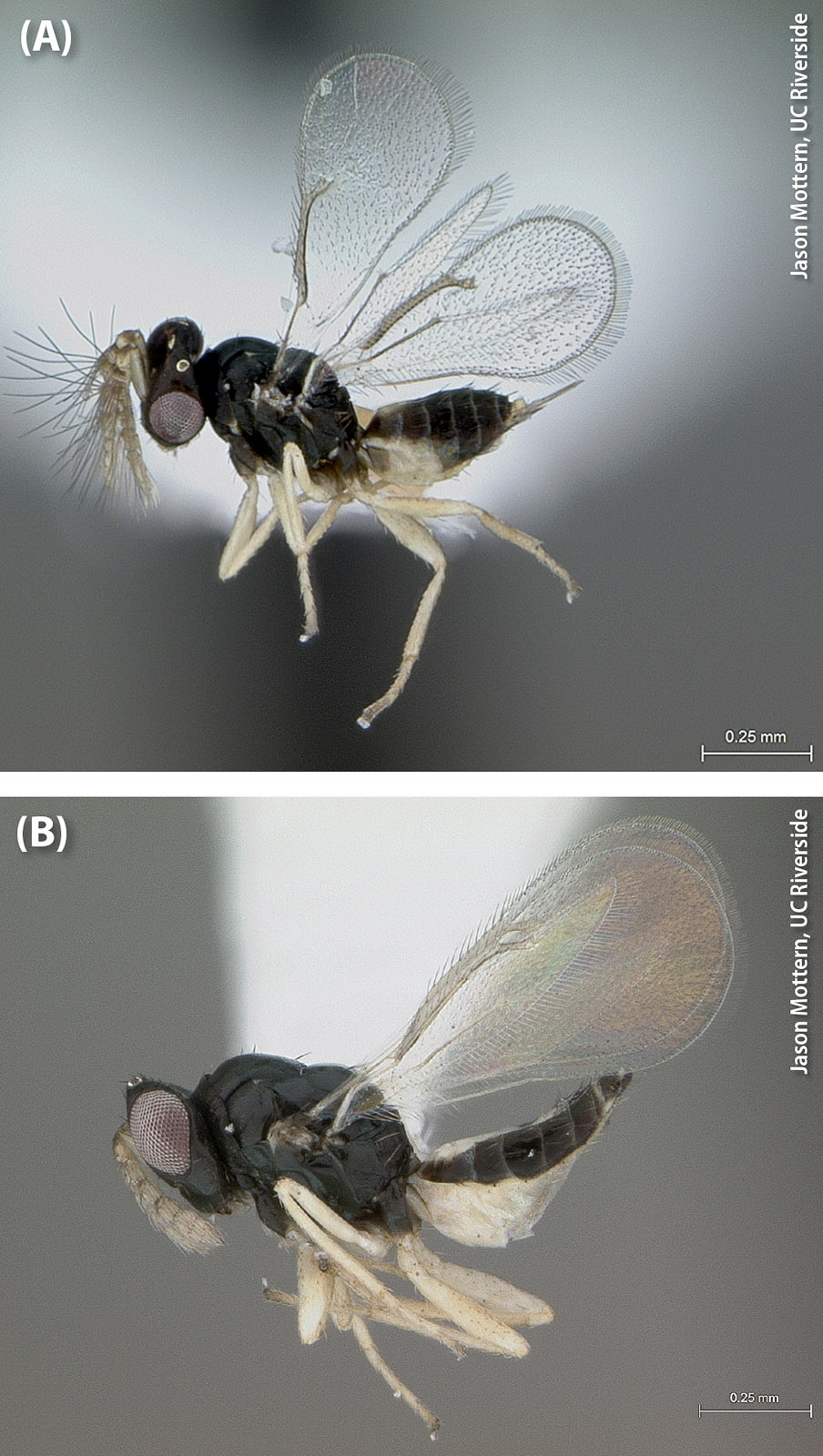
The second natural enemy species that has been released in California is D. aligarhensis, a host-specific solitary endoparasitoid of second through fourth instar ACP nymphs (Bistline-East et al. 2015). Like T. radiata, D. aligarhensis kills its hosts through a combination of parasitism and host feeding, and a single D. aligarhensis female can kill up to 280 nymphs in her lifetime (Rohrig et al. 2011). Releases of D. aligarhensis began in California in December 2014 (Vankosky and Hoddle 2016). By February 2017, over 300,000 D. aligarhensis had been released in urban Southern California by CDFA and UC Riverside (fig. 2C).
Adult Diaphorencyrtus aligarhensis (A) male and (B) female. Because D. aligarhensis parasitizes different ACP life stages than T. radiata, researchers are exploring the idea that the two species, which coexist in their native range, could complement each other in their attacks on ACP.
The concept underlying efforts to establish two ACP parasitoid species in California is that D. aligarhensis may complement T. radiata because the two parasitoids have preferences for different ACP life stages and both species coexist in their native range and contribute to ACP control in citrus (Khan et al. 2014). While it is uncertain at this stage as to whether or not T. radiata and D. aligarhensis will be complementary in their attacks on ACP, several other biocontrol programs targeting invasive pests of citrus in California have been successful because of established natural enemy complexes. The most notable are cottony cushion scale, Icerya purchasi (Maskell) (Hemiptera: Monophlebidae), suppression by the coccinellid Rodolia cardinalis Mulsant in hot desert areas, and the parasitic fly Cryptochaetum iceryae (Williston) (Diptera: Cryptochaetidae) in cooler coastal zones (Quezada and DeBach 1973).
The developmental biology of Diaphorencyrtus aligarhensis: (A) gravid female lays an egg inside the psyllid nymph; (B) in contrast to T. radiata, an adult D. aligarhensis emerges from the posterior of the ACP mummy. Studies of D. aligarhensis releases have not been completed yet, but early results indicate the species is able to reproduce in California urban citrus.
Releases of D. aligarhensis failed to establish populations in Florida. As seen previously with T. radiata, several factors may have led to this failure: (1) intensive insecticide use in citrus growing areas where D. aligarhensis was released (Rohrig et al. 2012), (2) the inferior competitiveness of D. aligarhensis compared to the widely established populations of T. radiata (Rohrig et al. 2012) and (3) the relatively low D. aligarhensis release numbers and frequency, which may not have been adequate to overcome establishment barriers.
Release and impact studies are still in progress, so it is too early to conclude that D. aligarhensis has established in California. However, evidence of D. aligarhensis parasitism of ACP has been found at over 85% of sites where this species was released. This finding tentatively suggests that D. aligarhensis can find ACP nymphs and is able to reproduce in California urban citrus, and that it may be able to coexist with T. radiata in this environment. Collectively, the two parasitoid species may intensify biocontrol of ACP in areas where they operate sympatrically.
Impacts of generalist natural enemies and invasive ants
California's biological control program targeting ACP has focused primarily on monitoring parasitoid establishment and their rates of spread and measuring levels of parasitoid-related mortality. However, a suite of naturally occurring generalist predators have been identified as important contributors to ACP mortality in urban areas. Studies conducted in Southern California have identified larvae of syrphid flies (Diptera: Syrphidae) and lacewings (Neuroptera: Chrysopidae) as members of this important predator guild (Kistner, Melhem et al. 2016). Field studies indicate that syrphid and lacewing larvae may account for ∼ 86% of ACP predation events, and mortality rates for experimental cohorts of ACP nymphs may reach 93% (Kistner, Melhem et al. 2016).
Exchanges between natural enemies and ants that disrupt natural enemy activity are highly relevant interactions affecting biocontrol success. Ants are widely recognized as natural enemy antagonists because of the food-for-protection mutualisms they form with honeydew-producing hemipterans (HPHs), like ACP. Many species of HPH are invasive, economically damaging pests (e.g., aphids, mealybugs, scales, whiteflies and psyllids) (Helms 2013).
An Argentine ant captures a foraging adult T. radiata. Argentine ants tend colonies of ACP nymphs for the collection of honeydew. Worker ants protect the nymphs from natural enemies, which can significantly reduce biological control of ACP. Research suggests that liquid poison baiting is an effective tool in reducing ant infestations in citrus and other managed agricultural systems.
In addition to directly protecting HPHs from natural enemies, ants disperse tended pests to new foraging areas and provide sanitation services to HPHs, which reduce the incidence of disease and honeydew drowning. Furthermore, tended HPHs may respond to ant presence by increasing their rate of phloem ingestion, resulting in more rapid development and higher reproductive output (Yoo and Holway 2011). Because ants may exacerbate existing pest problems, ant control is a critical component of integrated pest management (IPM) programs targeting HPH pests, particularly programs that rely on biological control agents for population suppression.
Given the considerable biocontrol efforts targeting ACP, there is a lack of research investigating the impact of ant–ACP mutualisms on natural enemy efficacy. Although studies from Florida found that ACP parasitism by T. radiata was substantially higher in groves where ACP-tending ant species were controlled (Navarrete et al. 2013), this interaction remains largely unstudied with ant species in California.
In Southern California, the most ubiquitous ant found in citrus is the invasive Argentine ant, Linepithema humile (Mayr) (Hymenoptera: Formicidae). This notoriously pestiferous and ecologically disruptive species has thrived in California for over a century. L. humile infestations are most extensive within managed environments, such as the citrus agroecosystem, because of irrigation and abundant food resources. In groves with heavy HPH infestations, a citrus tree may receive more than a million L. humile visits in a day (K. Schall and M. Hoddle, unpublished data). In a survey of urban citrus gardens in Southern California, L. humile was present in ∼ 90% of surveyed trees, with ∼ 55% of ACP colonies tended by workers. At these sites, ACP parasitism by T. radiata was significantly higher in citrus lacking L. humile (> 90%) than in citrus where L. humile was tending ACP colonies (< 12%) (Tena et al. 2013).
The results of replicated field trials in urban citrus groves in Riverside, California, further clarified the implications of the L. humile–ACP relationship for ACP biocontrol. ACP parasitism by T. radiata was 70% to 800% higher and generalist predators were ∼ 1 to 4 times more abundant in ACP colonies where L. humile was excluded or controlled, using a sticky barrier or liquid poison bait, compared to unmanaged populations of ants that had access to ACP nymphs (Schall and Hoddle 2017).
The high frequency of antagonistic interactions observed between L. humile and natural enemies of ACP is likely responsible for the disparities observed between treatments. For example, conflict with ants on citrus flush often resulted in prematurely terminated oviposition attempts by T. radiata, allowing ACP nymphs to escape parasitism. In some instances, ants were observed to capture and kill foraging T. radiata.
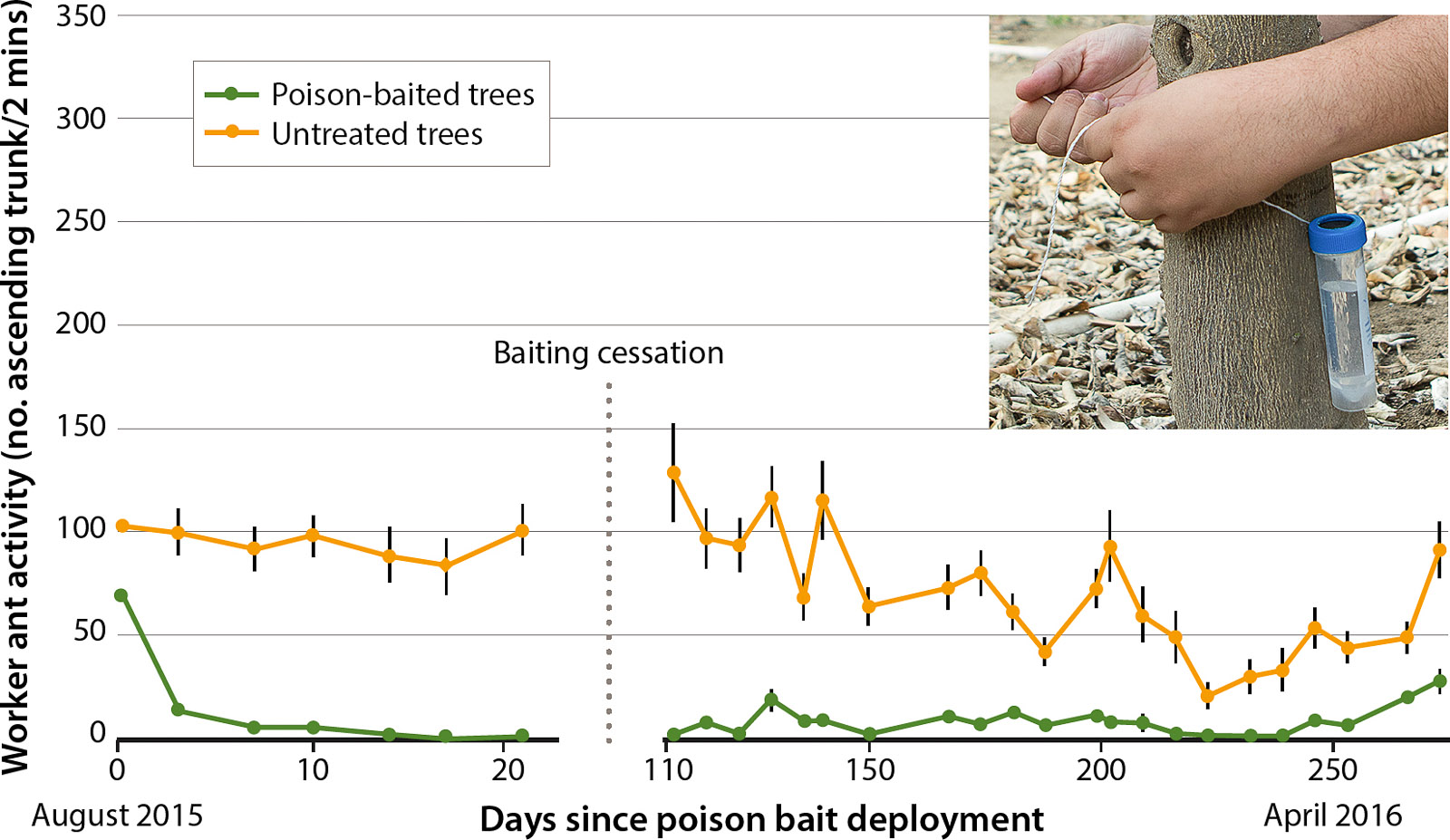
Together, these results suggest that L. humile can significantly suppress ACP biocontrol. Implementation of a liquid baiting regime for L. humile management is a highly effective method for improving biocontrol and reducing infestations of ant-tended HPH pests in managed agricultural systems (Cooper et al. 2008), including citrus (fig. 3) (Schall and Hoddle 2017).
Fig. 3. Visual two-minute assessments of Argentine ant activity on 33 liquid poison-baited (0.0001% thiamethoxam in a 25% sucrose solution) and untreated navel orange trees in an unsprayed citrus grove over a 9-month period. Activity on baited trees decreased by ∼ 75% within a few days of treatment and remained near 0 for the entire baiting period. Following bait removal in November, activity on previously baited trees was low and did not show signs of recovery until late April.
Future developments
It is anticipated that biological control of ACP in California will increase the efficacy and sustainability of other control strategies, including insecticide-reliant programs, because lower populations of ACP may need less management, and less frequent insecticide applications will slow the development of pesticide resistance. Reduced ACP populations resulting from natural enemy activity may also suppress rates of CLas spread and reduce economic losses resulting from HLB development in orchards.
However, ACP population suppression by natural enemies alone is unlikely to provide complete suppression of CLas spread in California. To reduce rates of CLas spread further, especially in commercial citrus production areas, IPM programs targeting ACP need to be developed that successfully incorporate a suite of complementary population control tools including natural enemies, ant suppression, insecticides and possibly the development of new citrus varieties tolerant of or resistant to ACP-CLas. Because ACP is significant within a complex of citrus pests, incipient management programs will need to be accommodating of well-developed control practices for other key citrus pests (e.g., California red scale) to minimize disruption of those existing programs.