All Issues
New controls investigated for vine mealybug
Publication Information
California Agriculture 60(1):31-38. https://doi.org/10.3733/ca.v060n01p31
Published January 01, 2006
PDF | Citation | Permissions
NALT Keywords
Abstract
The vine mealybug is a newly invasive pest that has spread throughout California's extensive grape-growing regions. Researchers are investigating new control tools to be used in combination with or as an alternative to standard organophosphate insecticide controls. Insect growth regulators and nicotine-based insecticides provide good alternative pesticides for use in some vineyards. Ongoing studies on the augmentative release of natural enemies and mating disruption also show promise, but commercial products are not yet available to growers.
Full text
In the early 1990s, the vine mealybug was accidentally introduced into the Coachella Valley (Gill 1994; Godfrey et al. 2003), probably from Mexican or Argentinian table-grape vineyards. This invasive pest quickly spread to grape-growing regions in the San Joaquin Valley (1998), Central Coast (1999), North Coast (2001), Sacramento Valley (2002), Sierra foothills (2002) and Monterey area (2002). As of fall 2005, the vine mealybug had been found in 17 California counties, and it is likely that more infestations have not been detected.
Vineyard mealybugs decrease crop quality by excreting honey dew, which promotes sooty molds, and by infesting grape bunches (Flaherty et al. 1992). The vine mealybug (Planococcus ficus [Signoret]) has biological characteristics that make it more damaging than other vineyard mealybugs (Godfrey et al. 2002). For example, the vine mealybug has a high reproductive rate, with some females depositing more than 250 eggs, and a fast development time, with four to seven generations per year in the San Joaquin Valley. In addition to producing abundant honeydew, the vine mealybug feeds on grape leaves and bunches through much of the summer. This pest also has a wide host range, which includes common weeds (such as malva), potentially increasing residual populations outside the vineyard. Finally, like other vineyard mealybugs, the vine mealybug can transmit grapevine viral diseases (Golino et al. 1999).
The nonnative vine mealybug, female (left) and winged male (right), excretes abundant honeydew, and infests and feeds on grape leaves and bunches. The authors investigated sustainable alternatives to conventional insecticides, which are often ineffective because the mealybug can reside under the bark.
Left to right, the vine mealybug was introduced into California in the early 1990s, and is currently found in at least 17 counties; infested windblown leaves can easily spread the pest from vine to vine, and vineyard to vineyard; grape bunches are rendered unmarketable.
Unfortunately, the vine mealybug is a pest that is here to stay in California and will likely continue to spread. Its dissemination is facilitated by the sticky nature of the honeydew; infested plant material may be moved by wind-blown leaves, animals, farm equipment and field crews. Grape clusters harvested from infested vineyards may also promote the pest's movement to new vineyard blocks because some mealybugs can survive the destemming process and grower-managed compost piles. As a result, caution must be taken with the disposal of harvest waste. Finally, because mealybugs often reside beneath the vine's bark or underground, the detection of infested nursery stock is difficult and the effectiveness of contact insecticides is reduced.
In response to the serious consequences of vine mealybug infestations in California, a consortium of University, private, and county and state personnel has initiated regional trapping and control efforts (Daane, Bentley, et al. 2004). Effective, insecticide-based control programs have been developed for regions where the pest is well established. In a series of studies, we focused on more sustainable controls that may work in combination with or as alternatives to standard insecticide programs.
Insecticide trials
Until recently, the recommended insecticide program for vineyard mealybugs was a delayed dormant organophosphate insecticide (chlorpy-rifos [Lorsban, Dow Chemical]) and/or in-season applications of a short-residual organophosphate (e.g., diazinon [Helena Chemical]) or carbamate (e.g., methomyl [Lannate, DuPont]) (Bentley et al. 2003). While these insecticides can provide adequate vine mealybug control, their repeated use may also kill natural enemies of the mealybug, reducing the level of biological control (Walton and Pringle 1999). We studied the effectiveness of two insecticides considered less disruptive than the organophosphates: imidacloprid (Admire, Bayer), a systemic, nicotinoid insecticide; and buprofezin (Applaud, Nichino America), an insect growth regulator. We report here on a part of this larger study.
Systemic insecticide
In 2002, we tested the effectiveness of imidacloprid as a systemic insecticide (applied through irrigation water and taken up by the vine roots) at different timings. The study was conducted in two vineyards, one with drip and the other with furrow irrigation, near Del Rey (Fresno County). The vineyards were mature (more than 20 years old) ‘Thompson Seedless’ blocks, planted in a well-drained, sandy-loam soil and managed for raisin grapes.
In each vineyard, imidacloprid was applied at full label rate in a randomized complete block with five blocks, each containing the following five treatments: 32 ounces imidacloprid per acre applied in (1) April, (2) May or (3) June; (4) 16 ounces imidacloprid per acre applied in both April and May; and (5) a no-insecticide control. Treatment plots were three rows by 80 to 125 vines (0.5 to 0.7 acres), running the length of each row. In the drip-irrigated vineyards, a 4- to 6-hour pretreatment irrigation prepared the soil. Imidacloprid was then applied through the irrigation system, and a 6- to 8-hour post-treatment irrigation was used to move the insecticide into the root zone. The furrow-irrigated vineyards were prepared by French plowing the berm and furrow area to expose surface roots, followed by a 1-day pretreatment irrigation. Imidacloprid was then applied into the furrows using an herbicide spray rig, and the application was followed by a 1-day posttreatment irrigation.
Mealybug density was monitored before treatment application (between March 13 and 19, 2002) by a field dissection of two spurs per vine on 25 randomly selected vines per plot for a total of 625 vines per vineyard (Geiger et al. 2001). To determine treatment effect, crop damage was evaluated at harvest using a O-to-3 cluster rating system, where 0 = no mealybug damage, 1 = honeydew (indicating the presence of mealybugs), 2 = honeydew and mealybugs but the cluster is harvestable, and 3 = unmarketable (Geiger and Daane 2001). In each treatment plot, 25 vines were randomly selected and nine clusters per vine were sampled.
Foliar treatments
In 2003, we tested five treatments in the existing 2002 drip-and furrow-irrigated plots. The five treatments were: (1) 32 ounces imidacloprid per acre, applied in May 2003 to plots that had received the same treatment in April 2002; (2) no insecticide applied in 2003 to plots that had received 32 ounces imidacloprid per acre in May 2002; (3) 12 ounces buprofezin per acre, applied in June 2003 to plots that had received 32 ounces imidacloprid per acre in June 2002; (4) 2 quarts chlorpyri-fos per acre, applied in February 2003 to plots that had received 16 ounces imidacloprid per acre in both April and June 2002; and (5) no-insecticide control plots (same plots as in 2002).
Because the 2002 plots were retreated, we conducted a more detailed spring survey to determine if the preexisting mealybug density would affect the 2003 treatments. Mealybug density was determined in March 2003 using a 5-minute search on each of five randomly selected vines per plot (Geiger et al. 2001). To determine the treatment effect, crop damage was evaluated at harvest, as described previously.
Natural enemy augmentation
As an alternative to insecticides, we investigated biological control of the vine mealybug. Natural enemies attacking the vine mealybug in California vineyards include the encyrtid parasitoids Anagyrus pseudococci (Girault), Allotropa sp. and Leptomastidea abnormis (Girault); several species of green and brown lacewings; and coccinellid beetles, including the mealybug destroyer, Cryptolaemus montrouzieri (Mulsant). Of these, Anagyrus is the most effective natural enemy of the vine mealybug in the San Joaquin Valley; as many as 90% of the exposed mealybugs collected near harvest time were parasitized (Daane, Malakar-Kuenen, et al. 2004). However, the parasitoid's effectiveness is hampered by at least four factors. First, from October to April vine mealybugs reside primarily underneath the bark, where they are protected from foraging parasitoids. Second, Anagyrus overwinter as immatures inside the mealybug and adults do not emerge until late spring, further reducing their early-season densities (Daane, Malakar-Kuenen, et al. 2004). Third, foraging ants protect mealybugs from parasitoids (Daane, Sime, et al. 2004). And finally, the parasitoid prefers larger mealybugs, especially for the production of female parasitoids.
We tested inoculative releases of Anagyrus as a possible mechanism to overcome some of these barriers. Field studies were conducted in five mature Thompson Seedless vineyards that were managed for raisin grapes and located near Del Rey. Treatments were Anagyrus release and a no-release control, with 1-acre treatment plots set in a randomized split plot design, and each vineyard serving as a replicate. Anagyrus were provided by the Foothill Agricultural Research (FAR) Insectary. We released 10,000 Angyrus per acre on June 12, July 3 and July 30, 2003, scheduled to occur when the mealybugs were in exposed locations on the vine (e.g., on the leaves).
Throughout the season, vine mealybug density was determined by 5-minute searches on each of 10 randomly selected vines per treatment plot. Mealybug numbers were recorded by development stage (e.g., first, second or third instar and adult). Parasitoid activity was evaluated by collecting 100 mealybugs from each treatment plot, which were recorded by development stage and location, categorized either as “protected” (e.g., underground or under trunk bark) or “exposed” (e.g., on leaves or clusters). When possible, we selected mealybugs in a one-to-one ratio from exposed and protected locations. The collected mealybugs were stored in gelatin capsules and held for parasitoid emergence, and then percentage parasitism and parasitoid species were recorded. Crop damage was evaluated at harvest using the cluster rating system (method described previously), with the exception that we sampled 50 randomly selected vines per treatment plot and five clusters per vine.
Disrupting mealybug mating
Until recently, a major hurdle in controlling mealybugs was the difficulty of detecting them in nurseries and vineyards. In 2001, a more effective monitoring method was developed utilizing the mealybug's sex pheromone. Female mealybugs, which are wingless, emit a sex pheromone to attract adult males, which have wings. This pheromone has been identified (Hinkens et al. 2001), synthesized and successfully used in monitoring programs (Millar et al. 2002; Walton et al. 2004). The synthetic sex pheromone's effectiveness and ability to be mass-produced led to our current studies on mating disruption of the vine mealybug.
We conducted studies in 2003 that used a microencapsulated formulation of the sex pheromone, applied to sections of five Thompson Seedless vineyards located near Del Rey, Sanger and Fowler (Fresno County). Treatments were pheromone applications (mating disruption) and a no-pheromone control, with 3- to 5-acre plots set in a randomized split plot design and each vineyard serving as a replicate. A 20- to 25-row buffer (330 yards) was used between treatment plots. The sex pheromone used was produced by Kuraray (Tokyo, Japan) and then microencapsulated by Suterra (Bend, Ore.). The pheromone was applied using an air-blast spray rig at a rate of 0.282 ounces active ingredient in 50 gallons of water per acre. Three applications were made in each field, with application dates between May 12 to 15, June 16 to 19, and Aug. 2 to 4.
Male mealybug flight was monitored using three Pherocon Delta IIID traps baited with sex pheromone lures (Suterra) in each treatment plot. Traps and lures were changed every 2 and 4 weeks, respectively. Mealybug density was determined using a 5-minute search on each of 10 randomly selected vines per treatment plot (method described previously). Crop damage was evaluated at harvest (method described previously), on 20 randomly selected vines per treatment plot and five clusters per vine.
The microencapsulated formulation starts emitting sex pheromone immediately after application and its longevity is dependent on temperature. To determine the field longevity, samples of pheromone-treated and clean (control) leaves were compared for their attractiveness to adult mealybug males. Ten leaves each were randomly sampled from pheromone-treated and control vines at 1, 7, 14, 21, 28, 35 and 42 days after pheromone application. The leaves were placed individually on the sticky surface of a pheromone trap, which was then placed 3.4 yards from a mealybug colony. After 24 hours, the numbers of adult males in traps with treated or untreated leaves were counted.
Statistical analysis
For all of these studies (insecticides, natural enemies and mating disruption), the results are presented as means per treatment (± SEM). Treatment impacts were compared using analysis of variance (ANOVA), with the means separated using Tukey's HSD test (P ≤ 0.05) for three or more treatments or using a t-test for two treatments. Treatment influences on cluster damage, as measured by the rating scale, were compared in a 2x2 contingency table with treatments separated using Pearson's chi-square (P = 0.05). Differences among specific treatments were evaluated as a series of pairwise comparisons, adjusting the critical value using the standard Bonferroni technique (P = 0.01). Repeated measures ANOVA analyses were used to determine season-long differences in mealybug densities, percentage parasitism and pheromone trap catches.
Alternatives to organophosphates
Systemic insecticide
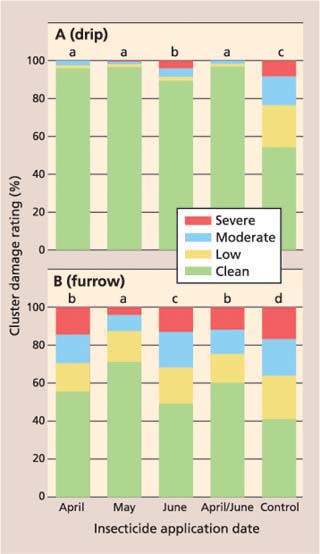
In the dripirrigated vineyard, there was a significant treatment impact on cluster damage (F = 1085.4, df = 12, P ≤ 0.0001). All treatments receiving imidacloprid, regardless of application date, had significantly less cluster damage than the control (fig. 1A). Average cluster damage ratings in the April, May and April/ June imidacloprid treatments were a significant 90.5%, 92.5% and 92.4% lower than in the control, respectively. Average cluster damage in the June imidacloprid treatment was a significant 67.9% lower than in the control, but significantly higher than in imidacloprid treatments applied earlier in the season.
In the furrow-irrigated vineyard, cluster damage ratings in all treatments with imidacloprid were significantly lower than the control (F = 221.58, df = 12, P ≤ 0.0001); however, there was a greater separation of the imidacloprid treatments (fig. 1B). Cluster damage in the May treatment was 59.3% lower than the control, and significantly lower than all treatments. Meanwhile, the April and April/June treatments were only 21.3% and 31.0% lower, respectively, than the control. The average cluster damage rating in the June application of imidacloprid was 14.8% lower than the control, but significantly higher than all other imidacloprid treatments.
The results show that imidacloprid provided the greatest reduction in cluster damage when applied in April or May through a drip-irrigation system, and was less effective when delivered through the furrow-irrigation system. We believe furrow-irrigated blocks have a more widespread root zone, which makes delivery of the insecticide to the entire root zone difficult and results in a more dilute application and poorer uptake of the applied imidacloprid. Irrigation both pre- and post-imidacloprid application is also critical, and this too is more difficult to properly manipulate with furrow irrigation.
It is important to note that these studies were conducted in the San Joaquin Valley on a sandy-loam soil; soil structure may change the efficacy of systemically applied materials. Imidacloprid and other systemic chloronicotinyls are moved with the irrigation water into the soil, picked up by the vine's root system, and then moved through the vine in its xylem. For this reason, proper delivery of imidacloprid may vary greatly among vineyards depending on soil and vine conditions. For example, there is evidence that the insecticide can bind with soil particles above the root zone when there is too little soil moisture, especially in heavier soils with higher clay content. In contrast, the insecticide may be flushed too quickly through and out of the root zone when too much water is applied in sandy soils. Once in the vine, imidacloprid must be delivered to sections where the mealybugs are feeding. Because all vineyard mealybugs are phloem feeders, there will be sections of the vine where the concentration and effectiveness of systemic insecticides vary (for example, the concentration of a systemically delivered insecticide may be higher in canes and lower in grape clusters). Researchers are currently investigating the uptake of systemic chloronicotinyls in the vine (N. Toscano, personal communication) and this information, developed for glassy-winged sharpshooter (Homalodisca coagulata [Say]), will greatly benefit mealybug control strategies.
Fig. 1. Percentage cluster damage ratings for imidacloprid and control treatments in (A) drip-irrigated and (B) furrow-irrigated vineyard. Clean = no mealybug damage; low = honeydew, indicating the presence of mealybugs; moderate = honeydew and mealybugs present; severe = unmarketable. Different letters indicate significant difference among treatments (P ≤ 0.01).
ystemic vs. foliar insecticides
In spring 2003, there were no significant pretreatment differences in mealybug densities among treatment plots in either the drip- or furrow-irrigated vineyards (drip: F = 0.922; df = 4, 145; P = 0.453; or furrow: F = 1.518; df = 4, 145; P = 0.200). Therefore, treatment impact was not obscured by pretreatment differences resulting from the previous year's insecticide application.
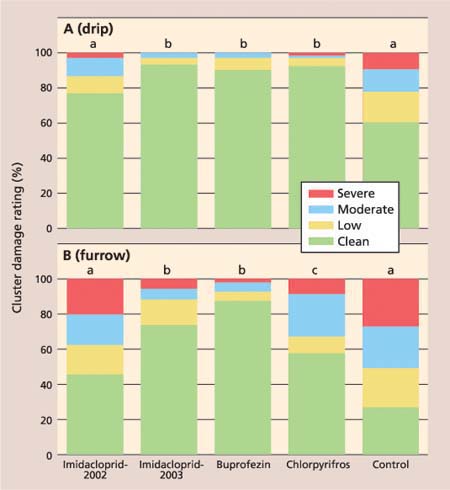
In the drip-irrigated vineyard, there was a significant treatment impact on cluster damage ratings (F = 221.58, df = 12, P ≤ 0.0001). Pairwise comparisons of the ratings for individual treatments were a significant 87.3%, 82.7% and 85.0% lower in the imidacloprid-2003, buprofezin and chlorpyrifos treatments, respectively, as compared to the control (fig. 2A). There was no difference between the imidacloprid-2002 application and the control. In the furrow-irrigated vineyard, there was a significant treatment impact on cluster damage ratings (F = 132.96, df = 12, P ≤ 0.001) (fig. 2B). The most effective treatments were imidacloprid-2003 and buprofezin, where cluster damage was 70.7% and 85.6% lower than the control, respectively. There was a significant 44.1% reduction in the chlorpyrifos treatment, whereas cluster damage in the imidacloprid-2002 treatment was not significantly different from that in the control (fig. 2B).
The fact that there was no significant difference between imidacloprid applied in 2002 and the control suggests that there was not an adequate year-to-year carryover of imidacloprid in the soil or root systems for vine mealybug control. The poor control achieved with chlorpyrifos in the furrow-irrigated block may be due to the location of the vine mealybug population in this vineyard, which had older vines (more than 30 years) that provided many protective areas under the bark of the trunk and spurs where mealybugs could remain hidden during much of the spring.
Control programs
Imidacloprid provided the greatest reduction in cluster damage when applied in April or May through a drip-irrigation system. We recommend that imidacloprid be applied near 70% bloom, typically from late April to mid-May. As noted, imidacloprid was less effective when delivered through the furrow-irrigation system. Even when properly timed (May) and delivered (pre- and postapplication irrigation), a single imidacloprid application did not locally extirpate vine mealybugs. In fact, the vine mealybug population recovered in all imidacloprid treatment plots between summer 2002 and spring 2003. This is presumably because imidacloprid cannot reach all parts of the vine, which leaves small pockets of mealybugs that can recolonize. Currently, ongoing investigations are determining the movement and concentration of systemically applied nicotenoid compounds, like imidacloprid, to different sections of the vine (personal communication, N.C. Toscano).
Fig. 2. Percentage cluster damage ratings for insecticide and control treatments in (A) drip-irrigated and (B) furrow-irrigated vineyard. Clean = no mealybug damage; low = honeydew, indicating the presence of mealybugs; moderate = honeydew and mealybugs present; severe = unmarketable. Different letters indicate significant difference among treatments (P ≤ 0.05).
Buprofezin provided excellent control, comparable to both imidacloprid and chlorpyrifos, and can be used effectively in vineyards with furrow-irrigation systems. We recommend that buprofezin be used as an alternative to in-season organophosphate treatments. Because buprofezin is an insect growth regulator, it is most effective on smaller mealybugs undergoing insect molts. For this reason, it will have greater impact when applied earlier in the season, before the mealybug population has overlapping generations. It will be least effective postharvest because late in the season (October and November) the development of most mealybugs has slowed and the population often consists primarily of adults and ovisacs.
Parasitoids help control mealybug
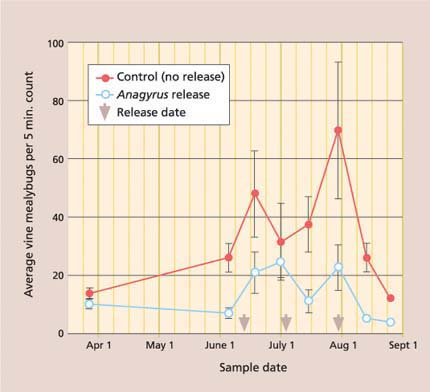
The season-long mealybug density was significantly lower in the Anagyrus release than in the control treatment (fig. 3). The average cluster damage rating was 57% lower in the Anagyrus release (0.22 ± 0.03) than in the control (0.51 ± 0.05) treatment (t = 5.52; df = 1, 444; P ≤ 0.001). However, we are unable to conclude that the released Anagyrus were solely responsible for this reduction. First, while there was no treatment difference in vine mealybug density on March 27 (t-test = 1.66, P = 0.101), which was when the treatment plots were randomly assigned, there were fewer mealybugs on June 5 (t-test = 3.70, P ≤ 0.001), which was just before the Anagyrus release. Second, there was no season-long difference in percentage parasitism (repeated measures ANOVA: F = 2.11; df = 1, 521; P = 0.15), although this is often an unreliable tool to measure the impact of natural enemies.
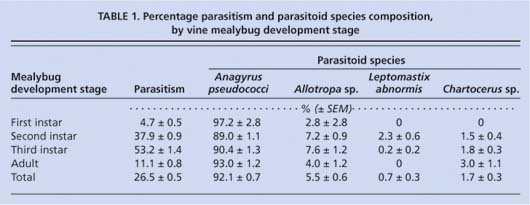
Nevertheless, the results provide encouraging information for the commercial use of Anagyrus to control vine mealybug. From 7,458 mealybugs collected and held in gelatin capsules, 1,978 were parasitized (26.5%) and 1,235 parasitoids were reared to the adult stage. The parasitoids reared were Anagyrus, L. abnormis, Allotropa sp. and Chartocerus sp. (the Chartocerus is a hyperparasitoid, which is probably attacking Anagyrus). Anagyrus was the dominant adult parasitoid, comprising more than 93% of the total (table 1). Third-instar mealybugs were most commonly attacked, reflecting the host preference of Anagyrus. Mealybug size affected the gender of the reared Anagyrus: first- and second-instar mealybugs yielded primarily males (100% and 83.3% ± 1.1%, respectively), whereas third-instar and adult mealybugs yielded primarily females (95.4% ± 1.1% and 92.9% ± 2.2%, respectively).
Fig. 3. Season-long average (± SEM) of settled (second instar to adult) vine mealybugs was significantly lower in treatments with Anagyrus pseudococci release, as compared to no-insecticide control plots (repeated measures ANOVA: F = 13.27; df = 1, 76; P ≤ 0.001).
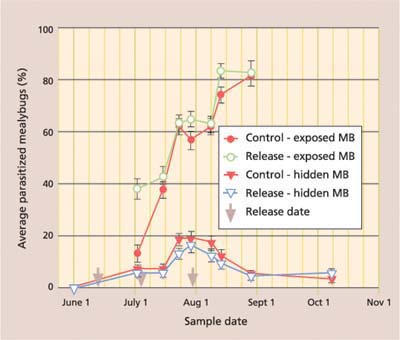
Season-long percentage parasitism, with data separated by date and location of collected mealybugs, shows the importance of timing augmentative releases after mealybugs have moved from protected locations (fig. 4). While the season-long percentage parasitism of mealybugs collected from protected locations (such as under bark) never exceeded 20%, there was a consistent season-long rise in parasitism of mealybugs collected from exposed locations (such as on the leaf). On the June 1 sampling date, which was prior to Anagyrus release, no mealybugs could be found in exposed locations. After releases began, there was a significantly greater percentage parasitism of exposed mealybugs in the release than in control plots on the initial sample (fig. 4). Parasitism rose steadily in both release and control plots because of the strong resident population of Anagyrus in this untreated field, reaching more than 80% by late August, after which we could find no live mealybugs in exposed locations.
Year-to-year declines
Resident Anagyrus are providing significant reductions in late-season vine mealybugs, which form the base for the following season's mealybug population. In fact, we have recorded a year-to-year decline in mealybug abundance in sampled vineyards near Del Rey. We are also enthusiastic about the commercial potential of Anagyrus and note the low rating for average cluster damage in the release treatment, which showed that an average of 78% of all clusters were clean and the remaining 22% had only minor honeydew damage.
TABLE 1 Percentage parasitism and parasitoid species composition, by vine mealybug development stage
Fig. 4. Season-long average (± SEM) percentage parasitism of settled (second instar to adult) vine mealybugs (MB), with data separated by treatment and location where mealybugs were collected. Season-long percentage parasitism was significantly higher in exposed than hidden locations for both control (repeated measures ANOVA: F = 247.3; df = 1, 273; P ≤ 0.001) and release (repeated measures ANOVA: F = 501.5; df = 1, 249; P ≤ 0.001) treatments.
The results of Anagyrus percentage parasitism and mealybug host stage preference will also help develop future release strategies. For example, most live mealybugs in the September and October samples were found in protected locations of the vine, such as under the bark. These protected locations greatly reduce the ability of foraging Anagyrus to locate and parasitize vine mealybugs. We believe this results in lowered parasitism levels of the overwintering mealybug population, leading to the observed low levels of Anagyrus the following spring (fig. 3).
Furthermore, we reared primarily male Anagyrus from first- and second-instar mealybugs. These results show that Anagyrus releases should be timed to coincide not only with the presence of mealybugs in exposed locations, but also with the presence of third-instar mealybugs, which are needed to support the production of female Anagyrus.
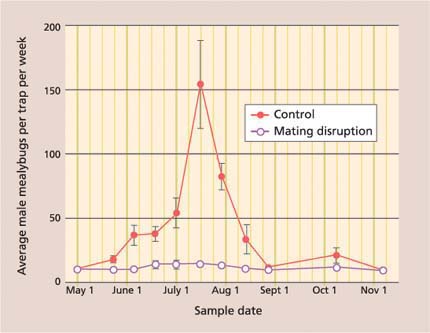
Fig. 5. Season-long average (± SEM) pheromone trap catches of adult male vine mealybugs in mating disruption and no-insecticide control treatments were significantly different (repeated measures ANOVA: F = 15.27; df = 1, 6; P = 0.008).
Mating disruption promising
One of the five vineyard blocks was partially treated with chlorpyrifos; this vineyard was removed from the data analysis and will be discussed separately. In the remaining four vineyards, season-long male mealybug trap catches were significantly lower in the mating disruption treatment than in the control (fig. 5). Essentially, pheromone traps were “shut down” in the mating disruption treatment because the vineyard was so inundated with sex pheromone that the vine mealybug males could not find the traps. More importantly, there was a significant reduction in crop damage in the mating disruption treatment (t-test: t = 5.76, P ≤ 0.001), with most of the clusters rated 0, or clean, and hardly any rated 3, or unmarketable (90.5% ± 1.5% and 1.2% ± 0.2%, respectively).
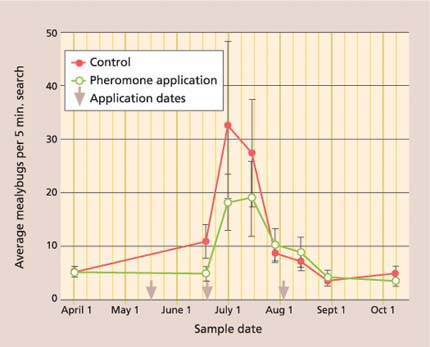
However, while crop damage was lower, season-long mealybug densities were not significantly different between the pheromone and control treatments (fig. 6). We believe that this may be explained by differences in mealybug location on the vine. For example, in the pheromone-treated plots, most live mealybugs were found under the bark on the trunk, while in the control they were under the bark, and exposed in leaves and clusters. We believe the between-treatment difference in mealybug location results from a beneficial artifact of the mating disruption: we consistently found higher parasitism rates of the exposed mealy-bugs in the mating disruption plots. This increase in parasitism levels in mating disruption plots has also been found in a recent South African study (Walton and Daane, unpublished data). Anagyrus may cue in on the mealybug pheromone and either remain in the vineyard aggressively searching for mealybug hosts, or be pulled in from nearby vineyards.
As mentioned previously, the cluster damage rating was lower in the mating disruption plots. Nonetheless, our research suggests that mating disruption may not be the most effective tool to quickly lower high-density mealybug populations. First, mating disruption works more slowly because it prevents the next generation from forming rather than killing the mealybugs already present. Second, mealybug density appears to influence the effectiveness of vine mealybug mating disruption.
In our trials, the overall level of crop damage was low even in the control treatment, with about 80% of the clusters clean (rated 0 or 1). This was in part by design, as earlier studies with mating disruption in heavily infested vineyards showed no treatment effect (Walton and Daane, unpublished data). We suspect that this may be due to the fact that at high mealybug densities, adult males would emerge in close proximity to females. Therefore, for these trials we selected vineyards with initially low or moderate mealybug densities. Still, there was even less damage in these vineyards than expected, considering that a few years before there had been nearly complete crop loss. We found that much of the mealybug reduction was the result of natural parasitism levels by Anagyrus. Clearly, the mating disruption program is quite compatible with biological control.
Fig. 6. Season-long average (± SEM) of settled (second to adult stage) vine mealybugs in mating disruption and no-insecticide control treatments were not significantly different (repeated measures ANOVA: F = 1.85; df = 1, 77; P = 0.18).
Currently, we are testing mating disruption programs that deliver the pheromone either as a microencapsu-lated formulation (provided by Suterra) or in dispensers (provided by Suterra, Shin-Etsu Chemical [Tokyo, Japan] and Scentry Biologicals [Billings, Mont.]). The advantages of the microencapsu-lated formulation include application using standard pesticide rigs, the dispersion of millions of microcapsules per acre to provide thorough coverage, and numerous point sources on each vine. One disadvantage, found in the 2003 study, is that pheromone activity was depleted after only 21 days; therefore, multiple applications per season are required. However, the longevity of product delivery (either in microcapsules or dispensers) is a technical problem that may be solved in product formulation. The advantages of dispensers are that they can be applied by hand, have the potential for longer activity (and so one or two applications per season), and have the potential for use in California certified organic farms.
Sustainable pest treatments
The vine mealybug is a serious pest that is here to stay. Along with its potential for damaging vines and reducing marketable yields, it often requires the considerable use of insecticides. While properly timed insecticide applications provide excellent control, their increased use runs counter to the grape industry's move toward sustainable farming methods.
Left to right, Jose Tinoco places a pheromone trap; Raksha Malakar-Kuenen, a postdoctoral researcher, samples for mealybugs; UC Riverside entomologist Jocelyn Millar and colleagues synthesized a vine mealybug sex pheromone, which has allowed for more effective monitoring.
Presently, organophosphates, nicoti-noids (imidacloprid) and insect growth regulators (buprofezin) are being used in vine mealybug control programs. Selection of the proper material or combination will depend on the time of year, mealybug density and vineyard condition (for example, imidacloprid may work best on sandy-loam soils). Selective insecticides, augmentation of natural enemies and mating disruption programs could provide growers with better tools to manage the vine mealybug.
Nevertheless, continued vigilance is needed to reduce populations and limit the pest's further spread. Growers should train all their workers in mealybug identification and react quickly to any new finds. Managers of infested blocks should follow all the recommended treatment protocols ( www.ipm.ucdavis.edu/PMG/r302301911.html ), and manage their equipment and workforce to minimize this pest's spread. Wineries should be aware of the status of vineyards delivering fruit and take steps to properly dispose of stems coming from infested blocks. Grapevine nurseries should implement quality-assurance measures to prevent the vine mealybug's further spread on plant materials.
By implementing appropriate control measures, the overall impact and dissemination of the vine mealybug will be reduced. Different regions vary in levels of vine mealybug infestation, and some regions may have compliance agreements in place for required treatments; growers should contact their local UC Cooperative Extension or county agricultural commissioner's office for information.