All Issues
Graft-transmissible agent causes bark necrosis and stem pitting in plum trees
Publication Information
California Agriculture 56(3):108-111. https://doi.org/10.3733/ca.v056n03p108
Published May 01, 2002
PDF | Citation | Permissions
Abstract
In two Central Valley plum orchards, nearly all the trees started exhibiting copious amounts of dark gumballs on scaffold branches and main trunks. Exposed bark showed extensive tissue necrosis and necrotic stem-pitting on the surface of the woody cylinders. Eventually, both orchards had to be removed and replanted. The symptoms were highly suggestive of a viral or viruslike disease agent. We began studies to characterize the pathogen associated with the failure of these orchards and were successful in associating the disease with a new virus that proved to have an extensive host range in many cultivated Prunus. Characterization of this virus is under way.
Full text
Scientists have recently characterized a disease first seen in 1986. Two Tulare County plum orchards succumbed to bark necrosis and stem pitting; tests confirmed a graft-transmissible agent. Symptoms include chlorotic ring spots, which were induced on ‘Shiro’ plum.
IN 1986, young ‘Black Beaut’ plum trees in two orchards in Dinuba (Tulare County) showed bark necrosis and gummosis on scaffold branches and the main trunk, and the woody cylinders were severely pitted. The disease was given the trivial name plum bark necrosis-stem pitting (PBNSP). The infectious nature of PBNSP was confirmed by graft-transmission assays performed at UC Davis (Uyemoto and Teviotdale 1996).
This situation occurred because scions of ‘Wickson’ plum (used as pollenizers) — that appeared to be healthy, but were in fact infected — had been whip grafted on every second-leafed ‘Black Beaut’ tree. All the grafts failed, but the disease agent was, nonetheless, transmitted into the trees.
Apparently, ‘Wickson’ plum is a symptomless host of the disease agent. Several Prunus species are grown commercially in California and others are used as virus disease indicators in the California Fruit and Nut Tree Registration and Certification Program (R&C Program). We were interested in determining host responses to this particular disease agent and providing descriptions of the symptoms. The hope is that our work will prevent further occurrences of the disease and provide diagnostic information to growers and advisors.
Test plant cultivars included:
-
Fay Elberta and GF 305 peach
(P. persica)
-
Bing and Mazzard cherry
(P. avium)
-
Colt cherry
(P. avium x P. pseudocerasus)
-
Shirofugen flowering cherry
(P. serrulata)
-
Mission almond
(P. dulcis)
-
French-Improved and Stanley plum
(P. domestica)
-
Angeleno, Black Amber, Black Beaut, Casselman, Friar, Laroda and Shiro plum
(P. salicina)
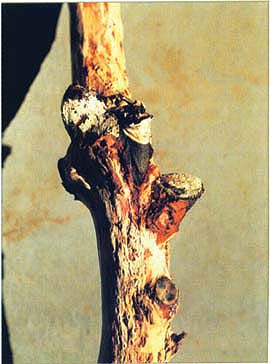
The authors tested a variety of plum cultivars for susceptibility to plum bark necrosis-stem pitting disease. A diseased ‘Black Beaut’ plum tree in bloom, 2 years after infection.
At UC Davis, we evaluated the responses of several Prunus species and varieties to infections by the PBNSP agent (see box). Two or three trees per variety were graft-inoculated in September 1997, with multiple bark tissues or peach bud chips. Trees that were not grafted served as healthy controls. The source of the PBNSP inoculum was diseased ‘Black Beaut’ plum trees on ‘Nemaguard’ peach rootstock, which produced numerous sucker shoots. The tree was located at UC Davis.
Disease symptoms induced
Following a 6-month incubation, chlorotic (yellow) ring spots were evident on young expanded leaves of ‘Colt’ cherry trees during spring growth. A year later, chronic symptoms consisting predominantly of light green or water-soaked marks developed along the leaf midrib. In other test plants, after 12 months of incubation, leaf symptoms on ‘GF 305’ peach and ‘Casselman’, ‘Laroda’ and ‘Shiro’ plums included chlorotic rings, chlorotic mottling and line patterns.
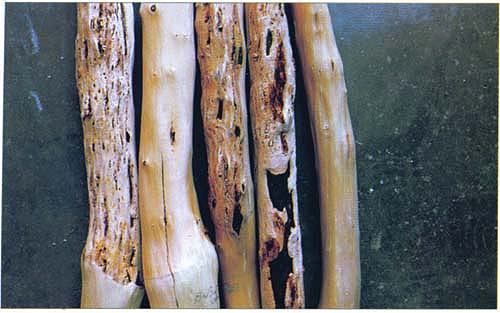
In the plum varieties ‘Angeleno’, ‘Black Beaut’ and ‘Friar’, gummosis (formation of gumballs) and bark necrosis (die-off of bark) begins in the second season after inoculations. Symptoms become more evident and severe in the third year. Wood markings intensified during the third growing season. There were no leaf symptoms on these plum varieties.
In contrast, latent (symptomless) infections occurred in ‘Fay Elberta’ peach; ‘Shirofugen’, ‘Bing’ and ‘Mazzard’ cherries; and ‘Black Amber’ and ‘Stanley’ plums. The trees were found positive by reverse transcriptase–polymerase chain reaction (RT-PCR) assays. ‘French Improved’ plum was apparently resistant to the causal agent. All healthy controls appeared normal.
Stem-pitting symptoms. Note remnant of grafting tape used to secure ‘Wickson’ scion piece. When the whip graft failed, a subtending ‘Black Beaut’ bud developed a shoot.
Several different types of leaf symptoms were identified on diseased trees. Leaf with chlorotic mottle on ‘Shiro’ plum.
Left and center, Leaves of ‘Colt’ cherry with light green and water-soaked marks along midrib, second year after inoculation. Right, A healthy leaf.
Leaf with line-pattern symptoms on ‘Laroda’ plum. Similar symptoms developed on ‘Shiro’ plum (not pictured).
With the single exception of ‘French Improved’ plum trees, virus infections in all of the other graft-inoculated test plants, with and without leaf or bark-stem symptoms, were positive. Healthy plants were found negative in a laboratory assay (such as RT-PCR) developed specifically for the associated disease agent.
Bark scraping from only symptomatic ‘Colt’ cherry trees yielded purified preparations of double-stranded ribo-nucleic acid (dsRNA). After electrophoresis in 6% polyacrylamide gels, the dsRNA preparations revealed a banding pattern similar to that reported for members of the Closterovirus group. Knowing this, we used purified preparations as templates for RT-PCR using degenerate primers designed specifically to detect the heat shock protein 70 (HSP70), a conserved gene found in Closterovirus (Tian et al. 1996).
When the reaction products from RT-PCR assays were evaluated using electrophoresis in 1.5% agarose gel, the presence of two DNAs was evident (Marini et al. 2002). The DNA bands were extracted and purified from the agarose gel and cloned, and desired DNA pieces were sequenced at the DNA Sequencing Facility at UC Davis.
The purification and cloning process followed the manufacturer's procedures that were supplied with standard kits.
Based on sequence information obtained, we designed new primers, and the primer combination yielded a specific product (541 bp [base pair]-size DNA) that was used in all RT-PCR assays of test plants.
Likely agents
In a previous publication, the PBNSF-associated virus was designated as PBNSPaV (Marini 1999). Absolute establishment of the causal relationship between PBNSPaV and disease symptoms in ‘Black Beaut’ plum would require fulfillment of Koch's postulates, often a difficult task in woody perennial plants such as Prunus; accordingly, Koch's postulates require the scientist to isolate and identify the agent, back-transmit the agent and produce the same disease on inoculated plants, and reisolate and reidentify the same agent. Nonetheless, the consistent association of the putative agent and PBNSP trees is highly suggestive of cause and effect. These observations suggest that ‘Wickson’ plum, like ‘Black Amber’ and ‘Stanley’ plums, may be regarded as another latent host for PBNSPaV.
At this time, the discovery of the Closterovirus PBNSPaV in stone fruit species can be regarded as something of a rarity. In the literature, only three others (Little cherry virus [LChV]-1, -2, -3) have been reported and all were found exclusively in cherry trees in Canada and Germany (Eastwell and Bernardy 2001; Rott and Jelkmann 2001). Sequence comparisons of the HSP70 gene revealed only 44% similarity between PBNSPaV and LChV-1, suggesting that these viruses are distinct and unrelated.
The extent to which PBNSPaV exists in California's stone fruit and nut orchards is not known. Nevertheless, widespread occurrence is not expected because ‘Shiro’ plum is routinely used as a standard indicator for Prunus virus diseases in the R&C Program. The presence of PBNSPaV in source trees destined for propagation purpose would be easily detected and avoided. Our research determined that ‘Shiro’ plum is a sensitive indicator for the putative agent that causes PBNSPaV disease.
’Shiro’ plum is a standard indicator used in the clean stock program; it was originally selected because of its sensitivity to diseased sources infected with line pattern-inducing agents, some of which are identified while others remain unidentified. Several unrelated viruses can cause line-pattern symptoms in ‘Shiro’. For example, in addition to American and Danish line-pattern viruses, we have associated line-pattern symptoms with a plum isolate of Prunus necrotic ring spot virus (Uyemoto, unpublished data). Nonetheless, in order to minimize serious virus-disease outbreaks in commercial orchards, growers should always request certified propagation materials identified in the R&C Program. This will to help ensure that virus-tested sources are collected and used in their orchards.