All Issues
Trace elements slowly accumulating, depleting in soils
Publication Information
California Agriculture 54(2):49-55. https://doi.org/10.3733/ca.v054n02p49
Published March 01, 2000
PDF | Citation | Permissions
Abstract
Certain trace elements are essential for humans, plants and animals, but become toxic at higher concentrations. For many trace elements, the margin of safety between beneficial and harmful is narrow. Deficiencies of trace elements are common plant nutritional problems in crop production. While most trace elements in soils are beneficial to plant growth, a buildup of trace elements may have a negative effect on whoever eats the plant. Trace elements may also degrade water quality downstream. Some are added to soils from the atmosphere, irrigation water and agricultural inputs including chemicals, biosolids, manures and compost. On cropland, important trace elements may also be depleted from the soil profile through leaching, crop harvest, surface runoff and volatilization. We calculated the levels of trace-element accumulation and depletion on the West Side of the San Joaquin Valley. Although the accumulation of chromium, cadmium, lead, mercury, nickel, copper and zinc on cropland will increase significantly over time, the rate of accumulation is slow and the added trace elements are not likely to interfere with farming in the foreseeable future. At the same time, arsenic, boron, molybdenum and selenium are being depleted from West Side soils. Elements removed in drainage water are now accumulating in evaporation ponds. To ensure desirable levels, water and soil sources of these elements must be monitored, and research into methods for limiting their accumulation and depletion should continue.
Full text
While naturally occurring, trace elements are redistributed in the environment by (clockwise from above) pesticide and fertilizer applications, industrial emissions, mineral extraction, agricultural drainage and other causes. Because soil is the starting point of the food-chain, the accumulation or depietion of trace elements can cause human disease.
Humans obtain energy and essential nutrients from food grown in soils and from animals that forage on plants supported by soils. The elemental profiles of plants and animals in a region resemble the geochemical composition of their habitats; elements are routinely transferred from the Earth's crust to the biosphere.
Over time, the impacts of trace elements on the metabolism of animals, plants and microorganisms have been delineated in detail. At the beginning of the 20th century, scientific evidence began to emerge that deficiencies and excesses of trace elements in soils had a profound impact on the well-being of plants and animals that depend on soil to thrive.
Excess or deficient trace elements have caused disease around the world. For example, symptoms of fluoride toxicity in sheep were observed in Iceland about a thousand years ago (Roholm 1937). Long-term exposure to food grown in selenium-deficient soils has for centuries caused the Kashan and Kaschin-Beck diseases — crippling bone disorders — throughout China (Atlas 1989). And millions of people who depend on the iodine-deficient soils of eastern Africa are still susceptible to goiter (Jaffiol et al. 1992).
Today, mineral extraction, industrial processing and consumer products bring us into daily contact with trace elements. Mine drainage, waste disposal, irrigation wastewater and urban storm runoff provide new pathways for the redistribution of trace elements in the environment, which can impact terrestrial and aquatic biota. Itai-Itai disease (a bone disorder caused by cadmium) and Minamata disease (a nerve disorder caused by mercury) were discovered in Japan in the 1950s. They resulted from chronic exposures through consumption of rice grown on cadmium-contaminated soils and fish tainted by mercury bioaccumulated through the aquatic food chain.
These incidences were classical examples of potential perils caused by trace-element pollution and demonstrated the environmental pathways through which pollutants could adversely affect human health (Kobayashi 1979; Harada 1979). In California, the reproductive failures and death of waterfowl caused by the bioaccumulation of selenium through the aquatic food chain in the Kesterson National Wildlife Reserve in California's Central Valley exemplifies the potential for ecological perils to occur on a much broader scale (NRC 1989).
Interpreted in the broadest sense, “trace elements” can encompass more than two-thirds of the 91 naturally occurring elements. We studied 11 which are likely to be significant in California soils. The following elements have the potential to impact terrestrial and aquatic biota, beneficially or adversely:
-
Boron (B), copper (Cu), molybdenum (Mo) and zinc (Zn) are essential for higher plants, terrestrial mammals and aquatic organisms and may become toxic at higher concentrations.
-
Chromium (Cr), nickel (Ni) and selenium (Se) are essential for metabolism in mammals and may become toxic at slightly higher concentrations.
-
Arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb) have no known vital biological functions, and are toxic to virtually all forms of life where they occur in available forms above critical levels.
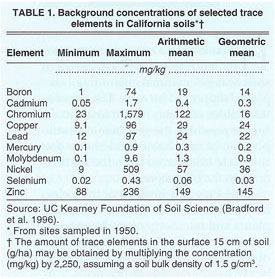
By and large, trace elements occur naturally in soils and are the weathering products of the parent materials that make up the Earth's crust. Because the processes of soil formation differ from place to place, the chemical forms, concentrations and biological availability of trace elements also vary widely. Soils in California are formed under diverse geological settings and have been influenced by drastically different soil-forming factors. As a result, the baseline trace-element concentrations of California soils vary considerably (table 1).
Trace elements in soils are subject to continual changes by the weathering process, which liberates those associated with insoluble primary minerals into chemical forms susceptible to further transformation. Water can mobilize the relatively soluble fractions in the soil and transport them to water bodies downstream, and vegetation can redistribute the trace elements. For example, trace elements can build up over time in surface soils in forests by plant absorption from subsurface layers and deposition of litter on the forest floor.
Human activities such as irrigation, fertilizer applications, crop harvests, atmospheric deposition from industrial emissions and automobiles, and waste disposal have altered the distribution and content of trace elements in soils. For many trace elements, the margin of safety between beneficial and harmful is narrow. The onset of an environmental calamity is often ambiguous and adverse effects may be hidden for years.
Removal of elements from soil
Although reasonable estimates for additions of trace elements to soils can be made, information on their removal is limited and location-specific. On cropland, trace elements may be removed from the soil profile through leaching, crop harvest, surface runoff and volatilization.
Leaching.
To demonstrate the balance between amounts of trace elements added to and removed from soils, we used data from tile drains on the West Side of the San Joaquin Valley to estimate removal by leaching. Leaching is the process by which elements in water percolate through soil to lower depths or are carried away by tile drains to evaporation ponds. The leaching volume and chemical, physical, mineralogical and biological properties of soil determine the extent that trace elements are depleted beyond the root zone.
Soils with fine texture and relatively large amounts of organic matter possess the greatest capacity to adsorb (assimilate by binding to the surface of soil particles) trace elements that are present in soil solution as positively charged ions (cations). Soils with relatively large amounts of the hydrous oxides of iron, manganese and aluminum have greater capacity to adsorb trace elements occurring as negatively charged ions (anions). Trace elements that are uncharged in the soil solution tend not to be adsorbed by soil constituents and pass freely with water that flows through the soil. Although other chemical forms may exist, cadmium, chromium, copper, mercury, nickel, lead and zinc commonly occur in soil solutions as cations, whereas arsenic, molybdenum and selenium occur as anions. Boron is unique in that it occurs as part of an uncharged compound.
TABLE 2. Estimated rate of removal for selected trace elements in soils of the San Joaquin Valley through subsurface drainage*
The characteristics of soil — (clockwise from left) clay, high organic content or sandy — determine the extent to which trace elements are depleted beyond the root zone.
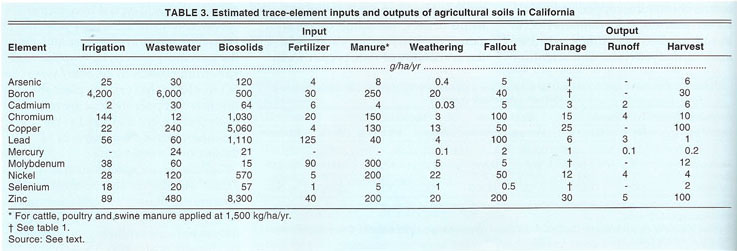
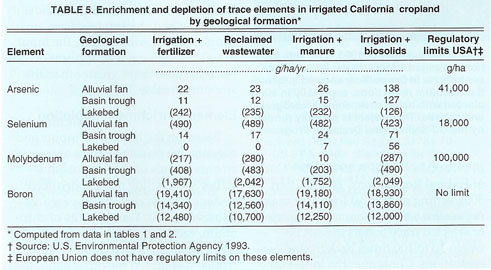
Concentrations of trace elements and volumes of subsurface tile drainage of irrigated fields on the West Side may be used to estimate the extent to which trace elements are leached from irrigated soils (Westcot et al. 1988; Tanji 1989). In San Joaquin Valley, the geologic setting (i.e., alluvial fan, basin trough or lakebed) influences the extent of soil leaching (table 2). For example, the annual depletion of molybdenum and boron is 1 to 2 orders of magnitude higher than that of arsenic and selenium, and ranges from 0.3 to 1.9 lb/ac (338 to 2,100 g/ha), and 15.3 to 21.6 lb/ac (17,000 to 24,000 g/ha), respectively, while the concentrations of cadmium, chromium, copper, mercury, nickel, lead and zinc were usually too small to quantify. Based on the limits of quantitation reported by Westcot et al. (1988) and on solubility considerations, estimates of annual leaching losses in drainage are small and range from less than 0.1 ounce per acre (<6 g/ha cadmium and lead) to 0.2 to 0.3 lb/ac (25 to 30 g/ha copper and zinc) (table 3).
Alluvial soils are derived from selenium-enriched ancient marine sediments of the Coast Range on the west side of the San Joaquin Valley, where the availability of state project water has resulted in agricultural development. In turn, irrigation and leaching led to the releases of selenium-laden drainage water. Soils occupying the basin trough and lakebed geologic setting are mixtures of sediments from both the Coast Range and Sierra Nevada and are much lower in selenium. Leachates from soils derived from all geologic settings are elevated in molybdenum and boron.
Vegetation.
The trace-element amounts removed by harvested plant matter can be estimated by concentrations in plant tissue and dry-matter yields. Alfalfa hay crops are harvested year-round in the Central Valley and produce a large biomass. As a result, an alfalfa crop is expected to remove larger amounts of trace elements from soils. We estimated removals by crop harvest based on an annual dry-matter yield of 3 tons/ac (6 megagrams/hectare [Mg/ha]) and typical trace-element concentrations in alfalfa hay (table 3). With the exception of copper and zinc, the amounts removed by crop harvests vary from <0.0009 to <0.09 lb/ac/yr (<1 to <100 g/ha/yr). The plant absorption of copper and zinc are relatively greater, but compared to the baseline levels of these elements for soils the amounts remain small.
Selected plant species such as Thlaspi caerulescens and Berkheya caddii accumulate unusually high concentrations of certain trace elements in their tissues without exhibiting phytotoxicity (Brewer et al. 1999). These plants, referred to as metal hyperaccumulators, have the potential to extract trace elements from contaminated soils. Most hyperaccumulators are not domesticated species and produce little biomass. In cultivation, they do not always respond to fertilization. Decades may therefore be required to remove significant quantities of trace elements from contaminated soils by this method.
Volatilization.
Chemical and biochemical reactions induced by bacteria and fungi in soils may convert arsenic, mercury and selenium in soils to the vapor phase. Selected plants are also known to absorb selenium from soil and volatilize it (Terry and Zayed 1994). These reactions, however, are sensitive to a variety of environmental factors. Unless the soils are manipulated to enhance the reactions, soil conditions are rarely optimal for substantial amounts of arsenic, mercury or selenium to be volatilized.
Elements added to soil
Atmospheric deposition.
The trace elements carried in the atmosphere come from diverse sources including volcanic activity and emissions from mining and industrial processing. Emissions from past mining and smelting activities have resulted in substantial buildup of trace elements on the nearby ground surface, with the amounts deposited decreasing farther from the source. More modern smellers are equipped with emission-control devices and tall stacks that reduce trace-element buildup in the soil.
Secondary smelting of scrap metals, industrial operations involving metal processing, and combustion of fossil fuels also may contribute trace elements to soil. Prior to 1975, lead was used in the United States as a gasoline additive; when gasoline combusted, lead was emitted in the automobile exhaust, causing lead enrichment of soils along the major traffic corridors in urban areas. Likewise, lead-based paints were common for decades, and as painted surfaces weathered lead was released and entered the soil. Because lead is practically immobile in soil, lead-contaminated surface soils remain a legacy in cities and along many highways.
While agricultural operations are normally not situated near metallurgical operations or along busy roads, atmospheric deposition from these sources may cover a wide area. To estimate the atmospheric fallout of trace elements, we rely on bulk deposition data in published reports (Galloway et al. 1982) and depositions computed from measured air concentrations and an assumed deposition velocity (Sposito and Page 1985) (table 3). This estimate represents the integration from all wet and dry deposition sources. Rates of deposition, depending on elements, range from 0.01 to about 3 ounces/ac (1 to about 200 g/ha) per year.
Irrigation.
All surface and ground waters contain small amounts of trace elements. Because 75% to 80% of California's water is used for crop irrigation, water can be an important source of added trace elements. To evaluate the extent to which irrigation contributes to trace-element increments in soils, we computed amounts added based upon an annual water application rate of 4 feet (1.2 m) and the typical trace-element composition of the San Joaquin River and reclaimed wastewater. Trace-element inputs from irrigation and from reclaimed wastewater applications are compared to input from other sources (table 3). Except for cadmium, lead and molybdenum, inputs of trace elements from irrigation water were greater than inputs from fertilizers. Depending on factors such as the crop and climate, annual applications of irrigation water in California vary and range from about 1.6 to 6.6 feet (0.5 to 2.0 m). We used a relatively high application rate of 4 feet (1.2 m), representative of irrigation of alfalfa, to correspond to our estimate for the removal of trace elements by a crop, in this case alfalfa.
Agricultural chemicals.
Trace elements may appear in fertilizers as supplements or contaminants. In addition, selected trace-element compounds have been used in insecticide formulations. The raw materials used to manufacture fertilizers, soil amendments and insecticides originate from diverse sources and contain varying amounts of trace elements, while the concentrations in these finished products also vary. Adriano (1986) showed that trace elements contained in nitrogen and potassium fertilizers are very low and are negligible compared to those in phosphorus fertilizers. We calculated the amounts of trace elements added from monocalcium phosphate applied at a rate of 54 pounds/ acre/year (60 kg/ha/yr) (table 3). Except for cadmium, lead and molybdenum, inputs from fertilizers are relatively small.
TABLE 5. Enrichment and depletion of trace elements in irrigated California cropland by geological formation*
Past uses of insecticides and herbicides containing copper, mercury and lead in orchards and vineyards, coupled with their immobile nature in soils, has led to substantial buildup of these elements in some areas where they were applied. Trace metal-based insecticides and herbicides have now been replaced by organochlorine and organophosphate pesticides. In apple orchards where lead-arsenate pesticides were repeatedly used, concentrations of lead and arsenic in surface soils were elevated by factors greater than 10 and 100 for arsenic and lead, respectively. Similar patterns of accumulation have occurred in old apple and citrus orchards that received copper herbicide applications. The use of mercury as an antifungal seed dressing has also contributed to mercury buildup in soils.
Biosolids, manures and compost.
Because of a ban on ocean dumping and tightened controls on incinerator emissions, biosolids are increasingly being applied to land. Biosolids, also called treated municipal sewage sludge, along with animal manure and municipal refuse compost, are used as supplemental fertilizers and soil conditioners on cropland. Because the trace-element concentrations of these wastes frequently exceed baseline concentrations in soils, their use can result in a substantial buildup of trace elements.
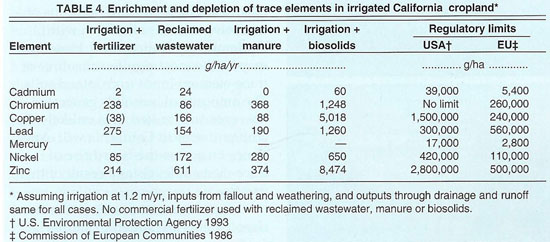
We have computed annual additions of trace elements based upon a moderate annual biosolids application rate of 4.5 tons/acre (10 Mg/ha) and the mean trace-element concentrations reported in a 1996 national biosolids survey (Pietz et al. 1998) (table 4). In 1993, the U.S. Environmental Protection Agency promulgated standards for land applications of biosolids (US EPA 1993, 1995, Code of Federal Regulations, Title 40, Part 503), setting numerical limits for maximum allowable contamination levels of selected trace metals in soils between 10 to 100 times background concentrations. Consequently, long-term, repeated land applications of biosolids may result in significant buildups of trace elements in soils. Even with moderate application rates, biosolids may be the most significant source of trace-element input to cropland soils. The amounts of biosolids generated, however, are limited. It is unlikely that cropland soils in California will experience an across-the-board rise of trace-element levels as a result of this practice.
At Tulare Lake Drainage District, a bloremediation treatment pilot project uses flow-through wetlands to reduce selenium concentrations in agricultural drainage water.
During the summer of 1998, selenium inflow averaged 13.5 parts per billion. Concentrations in the outflow showed substantial selenium reductions, especially in areas planted with baltic rush, smooth cordgrass and cattails. The project is partially funded by the UC Salinity and Drainage Program.
Except where trace-element mineral supplements are added to animal feeds, concentrations of trace elements in manure are less than in biosolids. When pig and chicken feeds are supplemented with copper, the input to soil through manure application can be 10 or more times greater than the amounts in table 3.
Element enrichment/depletion
Based on the balance of inputs and outputs, the transformation of trace elements in soils of the San Joaquin Valley's West Side may be deduced under common cultivation practices (tables 4 and 5). The amounts of chromium, cadmium, lead, mercury, nickel and zinc are expected to build up in cropland soils. On the other hand, the copper content of the soil is slowly being depleted under normal cropping practices. The extent of the metal accumulations in soils is low compared to the cumulative loading limits considered safe for crop production in the United States and by the European Community. Relative to the baseline concentrations in California soils, the enrichments of chromium, cadmium, lead, mercury, nickel and zinc are slight.
The greatest potential for trace-element buildup in cropland soils is in irrigated cropland where biosolids are used as a fertilizer. At present, reclaimed wastewater and biosolids are applied to only a few thousand acres in the San Joaquin Valley each year. Irrigation water and animal manures are also sources of chromium, cadmium, lead, mercury, nickel and zinc accumulation in cropland soils. In California, only about 7% of the cropland is required to accommodate all of the biosolids produced in the state. If the Central Valley bears its fair share of the land-applied biosolids, the cropland accumulation of chromium, cadmium, lead, mercury, nickel, copper and zinc would increase significantly, but the rate of accumulation is quite slow so trace elements are not likely to interfere with agricultural operations in the foreseeable future.
Despite the inputs from various sources, there is a net depletion of arsenic, selenium and molybdenum from cropland soils on the West Side, indicating the slow release of these indigenous elements. In the case of molybdenum and boron, the amounts dissolved from soils and transported to the tile drains are greater than amounts added through all input sources. For arsenic in soils developed from lakebed sediments and selenium developed on alluvium, the amounts appearing in tile drains are greater than the sum of inputs from all sources. Soils occurring in the alluvial fan and basin trough of San Joaquin Valley show a slight enrichment of arsenic, and those occurring in the basin trough and lakebed geologic settings show a slight enrichment of selenium. At present, where trace elements carried by agricultural drainage are retained along with salts in the evaporation ponds, we anticipate no immediate danger to downstream water bodies. Over time, arsenic, boron, molybdenum and selenium will be removed from the soil and will accumulate in evaporation ponds.
Future in focus: Meeting water-quality goals
Arsenic, boron, cadmium, chromium, copper, mercury, molybdenum, nickel, lead, selenium and zinc are trace elements of concern in California. These elements are inherent parts of soil, but they may also be introduced through irrigation water, fertilizers, soil amendments, waste disposal and atmospheric fallout. In the soil they may be removed by percolation into deeper soil layers, volatilization and plant uptake. Because soil is the starting point of the human food-chain, trace elements accumulated in or depleted from soils may cause nutrient deficiency or toxicity to growing plants, transfer of potentially toxic elements from soil to consumers, and degradation of downstream water quality.
Cadmium, chromium, copper, mercury, nickel, lead and zinc will continue to accumulate in the soil through irrigation water, fertilizer application and waste disposal. Under normal conditions, the rate of accumulation is slow and the accumulated elements are not likely to interfere with the use of the soils in the foreseeable future, even when wastes such as biosolids are applied on cropland.
Leaching accounts for a net depletion of arsenic, boron, molybdenum and selenium indigenous to soils of the West Side of the San Joaquin Valley and to similar soils elsewhere where agricultural drainage is collected and discharged. As leaching is inevitable for irrigated crop production, growers will need to discharge drainage water along with these elements for as long as the land is cultivated. Boron, molybdenum and selenium are essential for plant growth. The slow depletion induced by irrigation will some day render the soil deficient in these elements and the soils may need to receive trace elements supplements for successful crop growth.
If the collected drainage is discharged into water bodies, these elements may accumulate in the sediments and/or become biomagnified through the aquatic food web. If the discharge is retained in evaporation ponds, these ponds become temporary repositories of salts and trace elements removed from irrigated cropland. When the ponds are no longer used and dry out, mitigation measures must be devised to prevent these chemicals from breaching the containment barrier.
The harmful effects caused by trace elements tend to be chronic, gradually building up over continual exposures. As a result, potential ecological and human-health consequences related to the release of trace elements may go on unnoticed. Policies and regulations related to trace elements are being addressed at the federal level by requirements on meeting the ambient water-quality criteria and the basinwide water-quality management objectives (such as the Total Maximum Daily Load goals). Growers across the country will be expected to account for inputs and outputs of selected trace elements on the land they farm.