All Issues
Mowing and subclover plantings suppress yellow starthistle
Publication Information
California Agriculture 51(6):15-20. https://doi.org/10.3733/ca.v051n06p15
Published November 01, 1997
PDF | Citation | Permissions
Abstract
Yellow starthistle, a plant pest introduced to California in the mid-1800s, has infested more than 10 million acres and continues to spread. Vegetation managers, producers and land owners are searching for control methods that are compatible with their various land uses. Mowing and competitive plantings are two options that can be useful in yellow starthistle management programs. Timing is important. If mowing occurs too early, yellow starthistle can take advantage of the reduced competition for space, light and water. If it is done too late, large quantities of seed will disperse and replenish the seed bank. There were weed control benefits from planting subterranean clover as a competitive plant in combination with mowing, but the tested varieties declined substantially.
Full text
Left, early flowering stage, before seed maturation is optimum stage for first mowing. Inset, cross section of developing flowerheads. The flowerhead that is second from right (with faded flowers) contains mature seed.
Yellow starthistle is one of the most serious exotic plant pests in California. It was introduced in the mid-1800s and has now infested more than 10 million acres, according to the latest estimates. Yellow starthistle is toxic to horses, and its spines and ability to form dense stands impede access to the land. Because it grows in the summer and achieves high densities, it threatens the survival of native summer-active plants. The abundant biomass it produces increases the fire hazard at the end of summer. Despite its extensive distribution, it has not reached its geographical limits and continues to spread statewide.
The results of our earlier grazing experiments provided evidence that defoliation at later stages of growth (bolting, pre-spiny stage) did more damage to the plants and resulted in better control than defoliation at the earlier rosette stages (Thomsen et al. 1993). We became interested in mowing because it allows yellow starthistle to be defoliated at an even later stage than grazing, since the development of spines is not a limitation for mowing as it is for grazing. We anticipated that the effect of defoliation would be more severe when the plants were flowering. However, if mowing occurred in late flowering, the production and dispersal of viable seeds would prevent the long-term control of yellow starthistle.
Biocontrol researcher Don Maddox described the growth stages of yellow starthistle, Centaurea solstitialis L., and noted that yellow starthistle seeds become viable when the flowerhead fades, turning from a conspicuous bright yellow to a dull straw color. Therefore we targeted our late-season mowing to the stage at which the first bright yellow flowers appear but before any fading occurs. At this stage most of the flowerheads are still in the spiny bud stage and brightly pigmented flowers constitute a very small proportion of the total. We characterized this early flowering stage by sampling on June 10, 1992, at the Agronomy Farm site described in the next section. Out of a total of 1,800 flowerheads counted, about 98% were in the spiny bud stage, 1.4% were at the bright yellow stage, 0.5% were at the faded stage and only 1 (0.06%) had viable seeds.
Agronomy Farm experiment
A mowing experiment was carried out for 3 years (1992-1994) on annual grassland vegetation infested by yellow starthistle on the Agronomy Farm at UC Davis. We compared early-season and late-season mowing regimes for their ability to suppress yellow starthistle's reproductive output. We used a randomized complete-block design to control an existing gradient in the severity of the infestation.
The treatments were (1) a single early mowing at the bolting stage (late April to early May); (2) a single late mowing at the early flowering stage (mid-June); (3) late mowing at the early flowering stage, as in treatment 2, followed by a second mowing at early flowering on regrowth (late July to early August); (4) two late mowings as in treatment 3, but with manual removal of the yellow starthistle plants that regrew after the second mowing (in 1993 and 1994 only); and (5) unmowed control. Each treatment was represented in each of the four blocks. Treatment plots were 30 by 30 feet (9 by 9 meters) and were randomly assigned over the much larger treatment paddocks of our previous grazing experiment. Mowing was done with a flail mower mounted on a small tractor, and the mowed material was left in place.
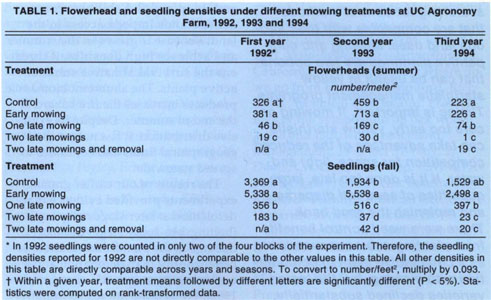
To evaluate the effect of the mowing treatments, we monitored yellow starthistle flowerhead and seedling densities. Flowerhead densities are an indicator of seed production per unit area, and seedling densities are an expression of the size of the seed bank. These densities were estimated by counting the number of flowers or seedlings that occurred in a 13.7-inch diameter (0.1m2) circular frame. We took 12 counts in each plot at locations determined in advance on a systematic grid. This sampling protocol satisfies randomization requirements while allowing us to directly compare seedling counts to flowerhead counts at each of the individual sampling locations. We sampled once for flowerhead densities in August–September and once for seedling densities after germination in November–December. Actual sampling dates depended on the regrowth after treatment and on the yearly rainfall pattern for seedling emergence. Each year we gathered 240 counts of flowerheads (5 treatments by 4 blocks by 12 samples) and 240 counts of seedlings, with the exception of the first year, when we sampled seedlings in only two of the blocks.
Our data involved small whole-number counts with many zero values in three of the treatments. Such data follows a Poisson distribution and violates the basic assumptions of analysis of variance. To remedy this situation, we transformed the data by ranking them before computing the ANOVAs, a practice established by Conover and Iman and implemented in the SAS statistical package. The treatment means of the rank-transformed data were compared by means of the classic LSD method. Although analyses were computed on rank-transformed data, the actual densities (number/m2) are reported to ensure a meaningful interpretation.
Initially, each year was analyzed independently of the others, and flowerhead densities were analyzed independently from the seedling densities of the same year (table 1). Next, the 3 years of data were analyzed conjointly, using the appropriate split-block design, to test for a year effect. We also investigated the relationship between seedling emergence and seed production by regressing the individual seedling counts on the corresponding flowerhead counts, using the 960 counts gathered in the 1993 and 1994 seasons. We obtained precipitation data from the weather station on the Agronomy Farm. Experiment-wide infestation levels by growing season were computed as the average of all flowerhead counts across all treatments, including the control.
Agronomy Farm results
Early mowing did not reduce yellow starthistle seed output and seed-bank size in any of the 3 years (table 1). In fact, early mowing in 1993 significantly increased flowerhead densities and subsequent yellow starthistle germination when compared to the control. Late mowing reduced yellow starthistle seed output and seedling densities in each of the 3 years. Plots that received a single late mowing had consistently lower densities of flowerheads and seedlings than the control. Each year, a second mowing provided an additional reduction in flowerhead densities when compared to a single late mowing. The seedling density of yellow starthistle in the two-late-mowings plots was significantly lower than in the single-late-mowing plots, except in the first year. Finally, manually removing the few yellow starthistle plants that survived two late mowings did not result in a significant reduction in subsequent seedling densities in the 2 years it was done.
TABLE 1. Flowerhead and seedling densities under different mowing treatments at UC Agronomy Farm, 1992, 1993 and 1994
An estimate of the number of seeds per flowerhead was obtained by random sampling of 48 flowerheads in the control plots. The number of seeds per flowerhead varied from 30 to 96, with an average of 54. Analysis of count data at the 240 individual sampling locations over 2 years showed a strong relationship between number of flowerheads and number of seedlings. Overall, flowerhead counts explained 72% (as measured by the regression R2) of the variation in subsequent germination at the same location.
Proper timing is important
This experiment demonstrates that proper timing is an important condition in the successful use of mowing in yellow starthistle management. Our results demonstrate that mowing can reduce but can also increase infestations, depending on when it is done. These contrasting results are consistent with those of our previous grazing experiments. Early grazing (at the rosette stage) by livestock increased yellow starthistle density, whereas late grazing (at the bolting, pre-spiny stage) significantly decreased flowerhead and seedling densities.
In this experiment, a single late mowing at the early flowering stage consistently reduced yellow starthistle's reproductive output. But a subsequent mowing, again at the early flowering stage, further reduced seed production. Although the first year's seedling numbers were not significantly different between the two-late-mowings and the single-late-mowing treatments, they were strikingly different the following 2 years.
The buffering effect of an established seed bank probably explains the lack of response noted in the first year. In support of this explanation, flowerhead numbers were significantly different between these two treatments right from the first year. Although most yellow starthistle seeds germinate within a year of dispersal, other investigators (Jolery et al. 1992) have demonstrated that a proportion of seeds can survive for several years in the soil. Therefore an initial reduction in flowerhead density and seed output may not be followed by a commensurate reduction in seedling emergence. To substantially reduce the level of infestation, it is necessary to reduce seed production consistently for several years.
The same buffering effect could also explain why 2 years of manual removal of the few plants that survived two late mowings did not significantly reduce seedling densities beyond those of the two-late-mowings treatment. Seed dispersal from adjacent plots might also be involved in this case, although Roche (1992) at Washington State University demonstrated that most yellow starthistle seeds fall within 2 feet of the parent plant. Based on these results, the complete eradication of yellow starthistle from a particular location is likely to require several years without any seed production or introduction.
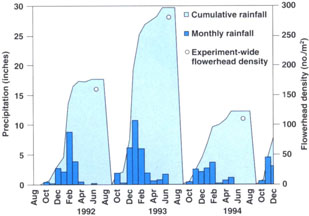
The conjoint analysis of the 3 years of yellow starthistle data reveals a highly significant year effect. Experiment-wide yellow starthistle flowerhead density was much higher in the Sept. 1992–Aug. 1993 growing season than in the other two seasons of experimentation, with 280 flowers/m2 in 1992–1993 versus 158 flowers/m2 in 1991–1992 and 108 in 1993–1994 (fig. 1). Average flowerhead densities in the control treatment alone (not shown) reflect the same trends.
Compared to the unmowed control on the left, early mowing increased yellow starthistle densities and subsequent germination.
Interestingly, the pattern of precipitation at the Agronomy Farm is very similar to that of starthistle densities (17.4 inches in 1991–1992; 29.6 inches in 1992–1993; 11.9 inches in 1993–1994; fig. 1). Furthermore, June 1993 was unusually wet, with 1.5 inches of rainfall. Other research has indicated that yellow starthistle's reproductive output is higher in years with greater amounts of rainfall (Sheley and Larson 1994). Our observations support these findings. Our hypothesis is that in wetter years, water accumulates in deeper soil layers accessible to yellow starthistle's deep taproot but generally not available to shallow-rooted annual grasses. Late-season rainfall replenishes soil moisture at a time when yellow starthistle is still growing, whereas most annual grassland plants have already completed their life cycles and died. Both these factors favor yellow starthistle summer growth and reproduction. By suppressing or removing competitors during a period when they are still intensely competing for resources, early mowing allows yellow starthistle to take advantage of the extra water. This would explain why in some years early mowing actually increases yellow starthistle infestation. For example, although “early mowing” in this experiment occurred at a later phenological stage than the “early grazing” in our previous work, the early mowing treatment still resulted in a significant increase in weed infestation in the wettest of the 3 years.
Planting clover and mowing
The mowing research at the Agronomy Farm demonstrated that timed mowing can significantly reduce yellow starthistle, but additional measures are needed to enhance control and prevent seed recruitment. Therefore the second experiment was designed to test the effectiveness of combining plantings of subterranean clover, or subclover (Trifolium subterraneum), and mowing to further suppress yellow starthistle.
The choice of a successful plant competitor should reflect the biological attributes of the pest plant, ecological conditions and the future use of the site. In the case of yellow starthistle, it appears that an appropriate competitive species should be capable of reducing light to yellow starthistle in the fall and winter during the seedling and rosette stages and/or use considerable soil moisture and nutrients in late spring and summer.
Subclover cultivars have been used for many years in California as a high-protein forage and as a nitrogen-fixing cover crop in orchards and vineyards. There are early-, mid- and late-season varieties that provide an opportunity for selecting cultivars for local climates and production systems. Characteristics that make some cultivars competitive include strong winter and spring growth, large leaf area, ability to recover after defoliation and flowers produced below mowing and herbivory heights.
In other research in which we tested dryland legumes as forage and cover crops, we observed that some subclover varieties suppressed yellow starthistle when stands were mowed in late winter or early spring. Subclover performance is enhanced by early mowing because the shading influence from tall annual grasses is reduced. Subclover regrows relatively quickly during this active growth period, and some varieties can form a dense network of interwoven stems and leafy canopies that reduce sunlight to associated yellow starthistle rosettes. However, yellow starthistle is deeper rooted and matures 1 to 3 months after subclover. Therefore relying on competition from subclover plantings alone is insufficient, and late spring or early summer mowings are required to further reduce the survival of yellow starthistle.
BIRC Field Station experiment
The second experiment is located at the Bio-Integral Resource Center (BIRC), 6 miles west of Winters in a foothill ecosystem. The soil is mapped as a Dibble-clay loam, a soil that is typical of pastures and occasionally orchards and dryfarmed small grains. The vegetation is dominated by yellow starthistle, annual grasses and lesser amounts of exotic and native forbs. We divided a portion of an undeveloped pasture into 12 15-by-25-foot plots for the following three treatments: (1) subclover seeding with mowing; (2) mowing with no subclover; and (3) control with no subclover seeding or mowing. The treatments were replicated four times in a randomized complete-block design. We prepared a seed bed by tilling the plots with a disk and harrow on June 13, 1993, to take advantage of a late spring rain that had moistened the soil. We disked the entire experimental area before any yellow starthistle seeds were produced.
On Oct. 9, 1993, we broadcast a 1:1 mixture of the subclover varieties ‘Koala’ and ‘Karridale’ at 40 lb/acre and lightly raked the soil to cover the seed. Prior to seeding, we inoculated the seeds with suitable legume bacteria and applied fertilizer — phosphorus at 100 lb/acre and sulfur at 136 lb/acre (repeated in 1995). A germinating rain occurred on Oct. 13.
Each year the seeded plots were mowed (3-inch mowing height) in February to enhance clover growth. These plots and the mowed-only plots were mowed yearly in June to defoliate yellow starthistle in the early flowering stage before any flowers had faded—that is, at the same stage of development described previously for the Agronomy Farm experiment. The first year these treatments plots were mowed again on July 24 to remove yellow starthistle regrowth and reduce seed production. Thereafter these plots were mowed only once in the summer (June) to allow us to better measure the effects of competition from the subclover.
Treatment effects were measured each year by sampling flowerhead densities with a 13.7-inch-diameter (0.1 m2) circular sampling frame to measure reproductive potential. Seedling densities were counted in November 1996 to measure the cumulative treatment effects on the seed bank.
BIRC Field Station results
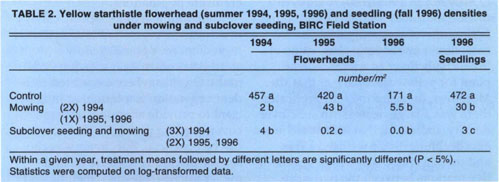
There were large reductions in yellow starthistle in both seeded and unseeded mowing treatments in each year (table 2). First-year results suggest that there was no additional yellow starthistle suppression in the subclover treatments. However, whatever competitive effect there may have been from the subclover the first year would have been negated by the second mowing in July, which effectively eliminated most of the yellow starthistle in both treatments. In the second and third years, 1995 and 1996, when we mowed only once in the bolting stage, better control was achieved in the subclover treatment than in the unseeded plots.
Second- and third-year results show a decrease in yellow starthistle in the subclover plots compared to the unseeded treatments. In the third year no yellow starthistle flowerheads were detected in the subclover plots. This decrease was also reflected in the yellow starthistle seedling density; there were 10 times the number of seedlings in the unseeded mowed plots as in the subclover plots.
To measure subclover regeneration, subclover seedling densities were counted in the third and fourth years of growth. Third-year subclover seedling densities were measured at 260 seedlings/ft2 (2,800/m2), and fourth-year densities were 80 seedlings/ft2 (860/m2). This is the equivalent of 172 and 53 pounds (194 and 60 kg/ha) of viable subclover seed per acre. This can be compared to the initial seeding rate of 40 pounds per acre.
BIRC Field Station discussion
The results demonstrated that there were weed control benefits from the subclover plantings. The vigorous growth and dense spring canopies produced each year by subclover helped suppress yellow starthistle growth. With fewer and presumably weaker yellow starthistle plant? remaining in the spring from the subclover competition, a single late-season mowing dramatically reduced yellow starthistle reproductive output the second year and completely eliminated seed production the third year. It appears that most of the yellow starthistle seeds in the seed bank here were eliminated in 4 years by a control program that allowed only minimal recruitment of new seed.
The results from the mowing without subclover seeding treatment are generally consistent with those obtained from previous work at the Agronomy Farm. Mowing yellow starthistle in the early flowering stage decreased canopy size and reproductive output and led to lower plant densities. Both sites support annual grassland species that probably provided some competition against yellow starthistle. In tall, dense stands of annual grasses, yellow starthistle seedlings etiolate and the rosettes have fewer leaves. Plants in the rosette stage tend to be upright rather than prostrate. Under these conditions, yellow starthistle is probably more susceptible to damage from mowing later in its life cycle than where there is less competition and more available sunlight.
Late mowing at the early flowering stage, followed by a second mowing at early flowering on regrowth, on right, significantly reduced flowerhead and seedling densities compared to the unmowed control on the left.
As other research has shown, the timing and amount of rainfall also influence the ability of yellow starthistle to regrow and reproduce. Therefore the degree of control that one can expect with mowing varies from season to season. Site-to-site differences such as variation in soil depth and water-holding capacity also affect yellow starthistle response to mowing. Some of these factors probably explain the differences in treatment responses between the sites. For example, two late-season mowings in unseeded plots the first year at the BIRC Field Station produced much better control in 2 of the 3 years at the Agronomy Farm under the same treatment. Much better control was obtained at the BIRC Field Station with one late-season mowing than with a single late-season mowing at the Agronomy Farm.
Subclover cultivar selection
The subclover mix ‘Koala’ and ‘Karridale’ selected for this study was based on the performance of 66 annual legume varieties (including Trifolium spp.—subterranean, rose, cupped, crimson, berseem, balansa and strawberry clovers; Medicago spp.—bur, barrel and snail medics; Vicia spp.—lana, purple and common vetches; Lotus purshianus—Spanish clover; and Lupinus nanus—lupine) tested in a sheep-grazed dryland pasture at the BIRC Field Station the year before this research began. The testing was initiated because BIRC personnel were looking for site-adapted legumes that could be used for weed control and forage and as a nitrogen source to enhance soil fertility. As a group the subclovers (26 were screened) far outperformed the other entries.
We selected the cultivars ‘Koala’ and ‘Karridale’ for the yellow starthistle experiment because they were among the top-producing entries (95% canopy cover at peak standing crop) the first year of the trial and seeds are commercially available. However, 2 years later they had both declined substantially (‘Koala’, 45% cover and ‘Karridale’, 30% cover) in the variety trial. Furthermore, in a second on-site cultivar screening in which the 12 best-performing subclover varieties were selected, ‘Koala’ canopy cover dropped from 95% to 5% while ‘Karridale’ was 70% in year 1 and 75% in year 3. Based on this result, our future choice of subclover cultivars would be different.
TABLE 2. Yellow starthistle flowerhead (summer 1994, 1995, 1996) and seedling (fall 1996) densities under mowing and subclover seeding, BIRC Field Station
Very few yellow starthistle seedlings grew in the treatment with two late mowings. Resident annual grasses dominate the plot.
Although the subclover seedling density was still higher than the first-year seeding rate, the decline in seedling densities from over 260/ft2 (2,800/m2) in 1995 to 80/ft2 (860/m2) in 1996 indicated a similar trend found in the cultivar screening trials, and it casts doubt on the long-term value of ‘Koala’ subclover as a replacement plant for yellow starthistle at that site. Moreover, in one of the four replicates there was a large decrease in subclover density, indicating that ‘Karridale’ was also declining. Since we used a mix and there were no distinctive vegetative markers between the two cultivars, it was difficult to determine which one had decreased the most.
Conclusion
Mowing can be a useful tool to manage yellow starthistle infestations in annual grasslands, if it is done during early flowering and repeated as necessary. If mowing occurs too early, yellow starthistle can take advantage of the reduced competition for space, light and water, especially in deep soils and wetter years. If it is done too late, large quantities of seed will disperse and replenish the seed bank.
Mowing at a stage similar to that of our early mowing treatment is common practice throughout California. Landowners and highway crews frequently mow when annual grasses mature and become a fire hazard. Mowing also removes grass and forb reproductive structures that attach to wool, fur or clothing and can cause injury to the eyes and ears of pets and livestock. Although early mowing may temporarily alleviate these problems, it can exacerbate yellow starthistle infestations and create denser stands that become a greater problem later on. Therefore successful management of yellow starthistle infestations by mowing depends on a specific “window of opportunity.”
Yellow starthistle response to mowing varies according to site conditions and climatic factors. Therefore, rather than relying on a fixed mowing prescription, monitoring followed by appropriate action is necessary to achieve satisfactory control. It should be anticipated that two or more mowings per year (at about 4-week intervals) will be needed for most sites and that mowing by itself will not eradicate populations.
Maintaining a dense spring canopy of associated vegetation is an essential ingredient for optimal yellow starthistle control in a mowing program. On sites where associated resident vegetation is minimal or insufficient to provide abundant spring cover, seeding plants for this purpose can be beneficial. Subclover was chosen for this study because of landowner interest, on-site screening and its history as a useful, noninvasive plant material in California agriculture. The BIRC Field Station results demonstrate that there were weed control benefits from the subclover plantings and fertilization when used with mowing, but the apparent decline of the subclover stand raises doubts about the choice of the varieties used here as long-term replacement plants. Nevertheless, yellow starthistle seed production has been consistently very low and was reduced to zero the third year. It appears that eradication may be achievable within several years if the mowing program continues and is supplemented with some spot manual control as needed.