All Issues
Electrostatic sprayers improve pesticide efficacy in greenhouses
Publication Information
California Agriculture 49(4):31-35. https://doi.org/10.3733/ca.v049n04p31
Published July 01, 1995
PDF | Citation | Permissions
Abstract
Electrostatic sprayers represent a new development in greenhouse pesticide application technology. In a 3-year study, we evaluated one of the newer candidate sprayers for efficacy in controlling green peach and melon aphids while enhancing worker safety. Electrostatic application provided aphid control that was equal or superior to conventional fullvolume spray while using 40 times less water in an equivalent area. In addition, although electrostatic application provided 3.7 times more foliar deposition than the use of conventional full-volume sprays, electrostatically deposited residues were more difficult to remove mechanically. Therefore, residues from electrostatic application are less hazardous to worker health and safety than conventional full-volume wet sprays.
Full text
Insecticides were sprayed using full volume and electrostatic application methods to control green peach aphid, Myzus persicae (shown here above and inset). Both methods reduced the number of aphid nymphs on potted chrysanthemum plants, but there was no significant difference between the two methods.
The application of pesticides in green-houses devoted to the production of ornamental plants is dominated by the use of fully dilute wet sprays under the historical concept of “spray to run-off.” It is common for applications to be in the range of 100 to 800 gallons per acre, depending on the type of crop, its stage of growth and the target pest. It has been documented that this is a grossly inefficient use of pesticides, with less than 1% of the active ingredient applied actually reaching the target pest. Despite this lack of efficiency, the ease, convenience and familiarity of this application method have made it routine among growers. In addition, pesticide labels consistently reinforce this application technology and have contributed to continued dominance of dilute sprays in the greenhouse.
This type of pesticide application is clearly inconsistent with the principles and practices of integrated pest management (IPM), which strive for conservative and efficient use of pesticides, where needed, with the absolute minimum amount entering nontarget areas.
Evaluation of alternative application methods is extremely complicated because of the diversity of target crops, the multitude of potential target pests and the variety of pesticides available for application. The evaluation process is further complicated by the introduction over the past 5 years of many new sprayers, each boasting numerous and varied improvements over conventional full-volume wet sprays.
One of the most intriguing new developments in greenhouse pesticide application technology is the use of electrostatic sprayers. These sprayers produce small droplets of highly concentrated pesticide, which are electrically charged as they leave the nozzle. These charged droplets penetrate foliage and adhere to all plant surfaces, including the undersides of leaves. Manufacturers claim that these sprayers provide excellent pest control while using very little water and reducing the total amount of pesticide needed.
Many of the claims for these sprayers may be true, but it is important to obtain objective data in the following areas: (1) efficacy in comparison to full volume wet sprays; (2) longevity of residues and potential hazards to the applicator; and (3) overall deposition in target and nontarget areas. For the past three years we have been evaluating one of the newer candidate electrostatic sprayers available to the greenhouse industry with respect to these areas.
New technology in old package
The electrostatic sprayer used in our evaluations (Electrostatic Spraying Systems, Inc., Watkinsville, GA) employs an air atomizing induction charging nozzle to produce droplets in the range of 30 to 60 microns. Within the nozzle, air pressure is equivalent to 30 to 40 psi, and liquid pressure is equivalent to 15 psi. (For comparison, a conventional hydraulic sprayer might require approximately 2,000 psi to produce droplets in this range.) Two 9-volt batteries impart a negative charge (approximately -6 mC/kg) on the droplets as they leave the nozzle. The technology used in this sprayer is a departure from earlier electrostatic sprayers, which were designed primarily for use in orchards.
The full-volume dilute spray applied to potted chrysanthemum plants to control aphids used approximately 130 gallons per acre.
A cooperating grower provided a site in San Mateo County for this evaluation. We used chrysanthemum plants, in 6-inch-diameter pols, that had just begun to set buds. Although there was a natural infestation of green peach aphid (Myzus persicae) on the plants, numbers were very low and there was too much interpot variation for a good test. Therefore we selected 4 pots randomly from each of the 12 benches in the trial, for a total of 48 pots. These pots were placed in a UC Davis greenhouse, where they were artificially infested with melon aphid (Aphis gossypii). Infestation was accomplished by placing clippings of infested chrysanthemum plants on the test plants and allowing 2 days for the aphids to move to the test plants. Before the pots were returned to the test greenhouse, a pretreatment count was taken, using a subsample of 12 pots. We cut the plants at the soil line, then washed off the aphids and counted them, noting the species and whether they were adults or nymphs. The remaining infested pots were returned to the test greenhouse and placed in exactly the same location from which they were taken. These pots were marked with red flagging tape.
At the time of this trial, the cooperating grower's choice of insecticide for aphid control was nicotine sulfate. We measured the amount of water that the grower typically used in a full-volume hydraulic spray at 30 gallons per 10,000 ft2 of bench; the rate of nicotine sulfate was 16 oz/100 gallons. In trials with water, the electrostatic sprayer used 0.67 gallons per 10,000 ft2 bench. To keep the amount of active ingredient the same per bench, we used 720 oz of nicotine sulfate per 100 gallons in the electrostatic sprayer. For comparison, these application rates are equivalent to 130 gallons per acre for the wet spray and 3 gallons per acre for the electrostatic spray.
A randomized design was used with four benches per application method and three pots examined per bench. Four days after the pesticide application, the test pots were returned to UC Davis; the same protocol used in making pretreatment counts was used in quantifying posttreatment aphid numbers.
Application of permethrin through the electrostatic sprayer was accomplished using approximately 3 gallons per acre. Residues deposited from electrostatic application were more difficult to remove mechanically than residues from the dilute spray.
Aphid control
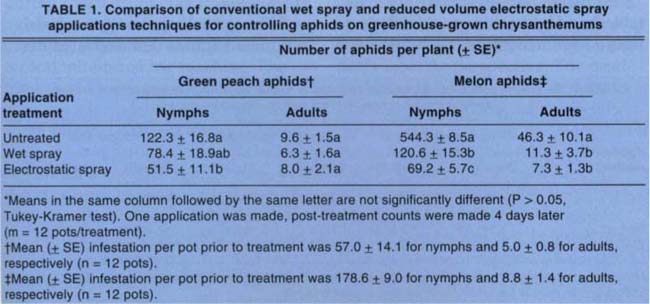
Both full-volume and electrostatic application methods significantly reduced numbers of green peach aphid nymphs compared to the control, but there was no significant difference between the two methods (table 1). Similar results were found with melon aphid adults. However, with respect to melon aphid nymphs, the electrostatic application provided significantly improved control over the full-volume spray. Although 100% control was not achieved, results reflected actual performance of nicotine sulfate in this commercial nursery.
TABLE 1. Comparison of conventional wet spray and reduced volume electrostatic spray applications techniques for controlling aphids on greenhouse-grown chrysanthemums
Deposition on nontarget surfaces
To test for deposition on nontarget surfaces, we chose a 1.6-acre commercial fiberglass/plastic-covered greenhouse located in Sacramento as the site. Chrysanthemums in 6-inch pots were used in the evaluations. The plants were grown on 6-feet-by-40-feet wooden benches raised 2 feet off the floor. At the time of the trial, plants were 8 inches tall with a leaf area (one side) equivalent to 700 square inches. Permethrin (formulated as Pounce 3.2 EC) was applied at the labelrecommended rate of 40 oz/acre, corresponding to 1 lb-ai/acre.
The conventional spray application was made using a commercially available sprayer (FMC Corp.) consisting of a variable cone nozzle integral with a high-pressure handgun to which liquid was supplied via a 100-foot hose from a two-cylinder piston pump operated at a pressure of approximately 300 psi. The application rate for the conventional sprayer was 250 gallons per acre, which is typical of a full-volume wet spray and consistent with the cooperating grower's normal application. Application of permethrin through the electrostatic sprayer was accomplished using 5 gallons per acre, which was 50-fold less than the conventional application. All applications were made by a full-time applicator employed by the cooperating nursery.
The application using both the conventional and electrostatic sprayers consisted of water, the permethrin formulation and Triton B-1956 surfactant (300 ppm by volume, as normally used by the nursery). The tank mix was buffered to a pH of 6.6 after mixing. Applications were made in the summer (June) and winter (December) of 1991. The application technique was similar for each spray system; the applicator walked along the bench at the rate of 1 foot per second while spraying. Each bench was sprayed from both sides, resulting in a typical application time of 70 seconds per bench, and each application method was used on four benches.
Foliar and nontarget deposition
Forty leaf punches, each 1 inch in diameter (63 inches2 total surface area), were randomly taken from the plants on each bench and placed in sample jars, which were sealed and refrigerated until extraction. All samples were analyzed within 24 hours of collection. Samples were taken 1, 3, 7 and 14 days after application. Pretreatment samples taken on all benches prior to application detected no permethrin. Permethrin was extracted from the leaf disk surfaces by using two 20-minute washes with 80 ppm of sodium dioctyl sulfosuccinate solution. Ethyl acetate was added to extract permethrin from the aqueous solution by filtering the solution through anhydrous Na2SO4. Aliquots were directly analyzed for permethrin on a Hewlett-Packard 5880A gas chromatograph; recovery for 20 μg of permethrin in the aqueous extract was 98%. Results reported are for the sum of cis-isomers and transisomers of permethrin.
We sampled deposition on nontarget surfaces using filter-paper dosimeters taped to the various surfaces. Each dosimeter exposed a 3.7-inches2 circle of filter paper. Dosimeters were placed on bench tops, in aisles and underneath benches. Dosimeters were removed 1 hour after application and analyzed for permethrin.
Foliar residue values were expressed as μg of permethrin per cm2 of leaf area, and nontarget deposition values were expressed as μg of permethrin per cm2 of surface area. All values were adjusted to compensate for variation in spray times during application by normalizing all data to a standard application rate of 3.42 grams permethrin per bench.
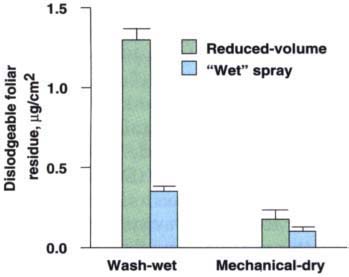
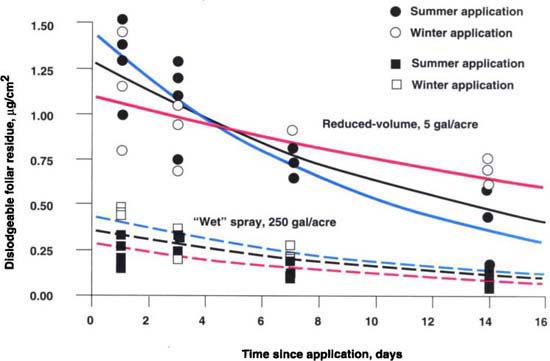
Fig. 1. Dislodgeable foliar residue as determined by the technique of Gunther et al. (1971) and mechanically dislodged residue for each application technique. Standard deviation shown by bars.
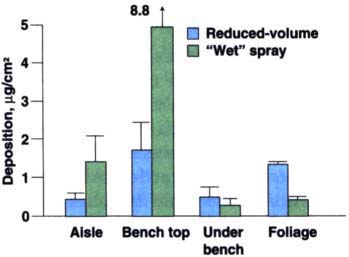
Fig. 2. Deposition on all sampled surfaces from each application technique. Standard deviation shown by bars.
The dislodgeable foliar residue data were used to analyze the rate at which the pesticide disappears from the plant foliage. Decay rate of pesticide residue depends on the pesticide characteristics, the environmental or climatic conditions and the target crop. This experiment was designed to determine the effect of application technique on the initial deposition and the decay rate. If the decay rate and the amount of initial deposition are known, then the amount of residue present at any time after application can be calculated. The decay rate of residue is most often expressed as the half-life, or time required for the residue to decrease by 50% of the beginning amount. Initial deposition amounts and half-lives were calculated and compared for winter and summer data for each application technique. Because reentry intervals and preharvest intervals are seldom adjusted for seasonal differences, these data were also pooled and analyzed.
Foliar residue and dissipation
Reduced-volume electrostatic application provided approximately 3.7 times more foliar deposition (as measured via surface extraction) than the use of the conventional full-volume wet spray technique (fig. 1). In addition, there was less deposition in the aisles and bench top from reduced-volume electrostatic application (fig. 2).
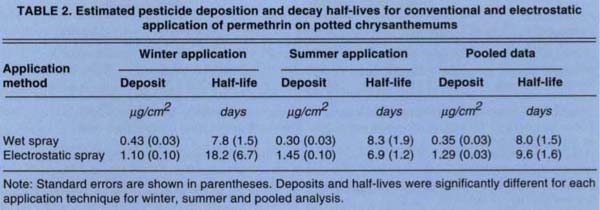
Estimated parameters are given in table 2 and presented graphically in figure 3 for analysis of the decay curves for each application method. Coincidence of the decay curves for the types of application was rejected, primarily because of the greater initial deposit from the reduced-volume application. Half-lives did not differ significantly for the summer and pooled data; for winter data, half-lives were significantly different (table 2). These results suggest that application technique could affect both the amount of initial deposition and the mechanical characteristics of the deposit. This conclusion was supported by the apparent interaction between weather and dissipation time of the electrostatically deposited permethrin.
TABLE 2. Estimated pesticide deposition and decay half-lives for conventional and electrostatic application of permethrin on potted chrysanthemums
Deposition from the conventional sprayer dissipated at the same rate for both summer and winter applications; electrostatic application dissipated almost twice as fast in summer as in winter. The experimental design employed in this trial allowed application technologies to be compared in a manner consistent with the intended commercial use; it did not allow any independent, individual assessment of the influence of concentration in the spray tank, spraying technique, and abiotic conditions (and their possible interaction) on selected spray characteristics measured in this study.
Discussion
Comparison of conventional and air-assisted, reduced-volume electrostatic pesticide application techniques found very different performance against aphids, amount of foliar deposition, residual decay over time and locations and amount of nontarget deposition of pesticide on plants and in the greenhouse.
Efficacy trials against green peach and melon aphids demonstrated that the electrostatic application provided control that was equal or superior to conventional full-volume spray while using 40 times less water in an equivalent area. This study was done keeping the pesticide rate per unit area of bench space the same. However, results from deposition studies suggest that because of superior foliar coverage (approximately 3.7 times more foliar deposition) using the electrostatic application, there is the potential to reduce the active ingredient applied by 3 to 4 times and still achieve the same amount of foliar deposition achieved with conventional spraying.
In the winter season, reduced-volume application resulted in significantly longer persistence of foliar residue. This may be viewed as positive for pest control; however, an evaluation of the possible impact on worker health and safety is of paramount importance. The extraction process reported here was done through standard washes, which is not an accurate measure of residues that workers may actually contact and remove. A measure of mechanically dislodgeable residues (brushing) provides a more realistic assessment of residue that workers may actually contact (fig. 1).
Studies using mechanical methods for dislodging residues conducted by UC in collaboration with the California Department of Pesticide Regulation, Worker Health and Safety Branch (A.M. Welsh, D.K. Giles and T.C. Blewett, unpublished), have demonstrated that only a fraction of the foliar residue that can be removed by standard washing techniques may actually be available for transfer to workers during commercial operations.
Mechanical removal is assessed by brushing whole-leaf samples using a typical mite-brushing machine. The removed residue is captured on a filterpaper surface beneath the brushes. A vacuum is maintained across the paper and air is pulled across the rotating brushes and through the filter paper. Residues deposited from electrostatic application were more difficult to remove mechanically than residues from a dilute wet spray (fig. 1). Over 30% of the surface residue on the leaf after wet spraying could be removed by mechanical brushing. Less than 14% of the residue from electrostatic spraying could be mechanically removed.
Fig. 3. Dissipation of dislodgeable foliar residue (by washing) from conventional and reduced-volume applications in summer and winter. Blue lines are decay curves for summer applications, red lines are decay curves for winter applications and black lines are decay curves for pooled data.
Although these data may satisfy some concerns about the safety aspects of using electrostatic applications, additional research is needed in these and other areas. This is particularly true when considering the number of different pesticides that might potentially be applied using this application technique. Two major concerns are applicator exposure and spray concentration. If the spray is directed away from the applicator, there is little problem of contact because the charged droplets are attracted to the plants where the spray is directed.
However, the higher concentration used in the spray tank of electrostatic and other low-volume, high-concentrate sprayers is a problem from a pesticide labeling perspective. The pesticide label is the law. If a statement is made on the label regarding specific dilution rates (for example, if the label states “Apply in at least 100 gallons of water per acre”), and the label does not otherwise suggest the use of “concentrate sprays,” then it is illegal to use the pesticide in concentrate sprays because it is inconsistent with the label.
It is ironic that greenhouse labels that specify a given rate of pesticide per 100 gallons of water often include no recommendation about how much water should be used per acre or unit of bench area. In essence, these labels allow legal application of an unlimited amount of pesticide active ingredient per acre, but forbid use of reduced-volume sprayers that may apply greatly reduced rates of pesticide active ingredient.
The labels of many newer pesticides registered for greenhouse use include directions for use in reduced-volume (“concentrate”) sprayers and foggers. The future “fast-track” registration process for bio-rational pesticides may allow more efficient application techniques. Currently, the greenhouse industry, UC and the California Department of Pesticide Regulation are working cooperatively to address the regulatory issues. In the meantime, growers who wish to use reduced-volume application systems are limited to pesticides labeled for that use.