All Issues
Insect pathogen “Bt” controls peach twig borer on fruits and almonds
Publication Information
California Agriculture 47(5):4-6.
Published September 01, 1993
PDF | Citation | Permissions
Abstract
Early season sprays of the insect pathogen Bacillus thuringiensis, or “Bt,” were applied to peaches and almonds to determine efficacy against peach twig borer (PTB). Data indicates one or two Bt treatments during bloom are as effective as a dormant organo-phosphate plus oil spray for PTB control. Research is currently under way to determine the impact on other pests.
Full text
The peach twig borer (PTB), Anarsia lineatella, is a serious pest of many tree crops grown in California. Hosts include peaches, nectarines, plums, prunes and almonds. For many years the recommended control for PTB has been to treat overwintering larvae with an oil plus organophosphate insecticide. In addition to being effective for other pests such as San Jose scale, a dormant treatment is considered relatively nondisruptive to biological control. Recently, growers and consultants have reported dormant treatments less effective for PTB control than in the past. In addition, dormant treatments have come under scrutiny as the sprays are suspected of drifting to nearby crops and environmentally sensitive areas. They have also been implicated in killing raptors, including hawks, present in orchards at the time of application.
First instar PTB larvae construct overwintering sites, called hibernaculae, beneath the bark in the crotches of 2- and 3-year-old wood. Some PTB begin overwintering as early as August, and the remainder enter these chambers as the season progresses. Larvae feed within the hibernaculae on warm days during the winter and emerge as first or second instars as trees begin to leaf out. After emerging, these larvae feed on new shoots and fruit buds, often as many as five or six, before maturing. Eventually, as shoots elongate, enough soft tissue is present for larvae to tunnel into and complete development in them. After completing their development, mature PTB larvae seek sheltered areas to pupate. They emerge as adults in late March or early April and lay eggs. These eggs, upon hatching, produce the first summer generation. Larvae of the first summer and subsequent generations complete their development inside a single shoot or fruit. There are three or four generations a year.
Control of the first summer and later larval generations with stomach poisons or insect pathogens has not been successful because these larvae emerge from eggs and immediately bore into shoots or fruit, failing to ingest a lethal dose of the toxic material. Overwintered PTB, however, are vulnerable to such materials because they emerge as first and second instar larvae and feed on several different shoots before maturing.
This paper describes our strategy to control overwintered PTB larvae using the Kurstaki strain of Bacillus thuringiensis (Bt), an insect pathogen affecting only lepidoptera larvae. In addition to being selective, Bt is regarded as nontoxic to bees, humans and wildlife, and is considered safe for the environment. Experiments were initiated to determine the potential for Bacillus thuringiensis (Bt) to control PTB as an alternative to traditional organophosphate dormant treatments. These experiments were conducted during the spring of 1990 and 1991 in a peach and nectarine orchard near Kingsburg (Tulare County), and an almond orchard at the Nickels Soil Laboratory near Arbuckle (Colusa County).
Peach and nectarine study
Three-year-old ‘Elegant Lady’ peaches and ‘Sweet Home’ nectarines were treated from one to five times and at different combination of timings with 2.4 pounds per acre of Javelin WG, a commercial formulation of Bacillus thuringiensis. Eighteen combinations of multiple applications and treatment timings were applied. For comparison, a standard dormant treatment of 3 pounds formulated diazinon 50 WP plus 4 gallons of oil per acre was applied on February 20 and some trees remained untreated as a control. Treatments were arranged in randomized complete blocks with four single tree replicates in peaches and three in nectarines. Treatments were applied by handgun sprayer using 2 gallons of material per tree. The trees were spaced 16 x 18 feet apart, resulting in 150 trees per acre.
Followup work has indicated that aerial sprays of Bacillus thuringiensis are not as effective as ground applications; coverage of leaves is insufficient and insect pests do not ingest enough Bt.
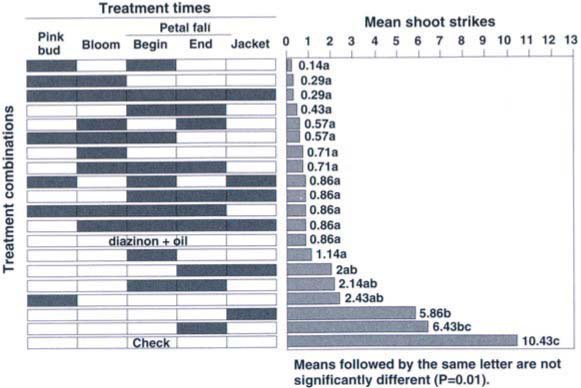
The first Bt treatment was applied March 1, when trees were at early pink bud and shoots were 0.5 inch long. Applications were repeated according to our design with different combinations of timings at 7-day intervals until after jacket split when shoots were 3 to 6 inches long (fig. 1). Rain fell during the early part of the period, with 0.10 inches March 3 and 0.24 inches March 12. No rain fell after March 12.
The treatments were evaluated by counting all shoot strikes April 4. This was before the beginning of the first adult PTB flight. Oriental fruit moth (OFM) was controlled by mating disruption and few, if any, OFM shoot strikes were present in the orchard when PTB strikes were counted.
Fig. 1. Impact of Bacillus thuringiensis by treatment time on Anarsia lineatella in nectarines and peaches in Tulare County, 1990.
Almond study
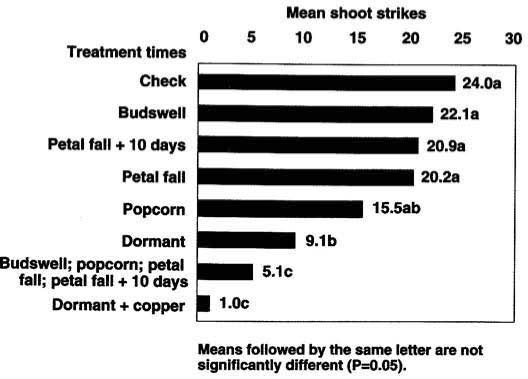
In 1990, second-leaf ‘Butte’ cultivar almonds were treated with 0.75 pound per 100 gallons of Javelin WG at four different treatment timings and one treatment at all four timings for a total of five treatments. The first treatment timing was at bud swell. Others were applied at 10% bloom, petal fall and early leafing stages. Included also was an untreated control and, for comparison, standard dormant treatments of 1 quart formulated Supracide 2E plus 1 gallon oil per 100 gallons and Supracide 2E plus oil with copper added were applied February 20. Treatments were arranged in randomized complete blocks with 10 single tree replicates. Sprays were applied to runoff at approximately 220 gallons per acre or 1.5 gallons per tree, before and after bud break.
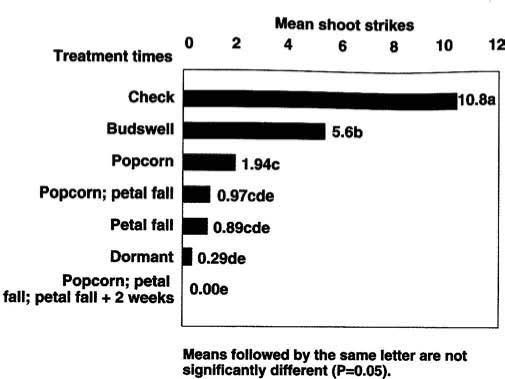
This study was repeated in 1991 on third-leaf ‘Butte’ cultivar almonds to reevaluate the treatment timings and to test additional combinations of treatment timings. For comparison, 1 pound formulated diazinon plus 1 gallon oil per 100 gallons was applied January 24. Untreated trees were used as controls. Treatments were arranged in a randomized complete block with seven single tree replicates. Dilute sprays were applied to runoff at 300 gallons per acre.
Treatments in both years were evaluated in April before the development of first summer generation larvae by determining the total number of larval entries (strikes) of shoots per replicate. Treatments for both crops and within years for almonds were compared by analysis of variance, and means separated by Duncan's new multiple range test.
Fig. 2. Impact of Bacillus thuringiensis by treatment time on Anarsia lineatella in almonds in Colusa County, 1990.
Fig. 3. Impact of Bacillus thuringiensis by treatment time on Anarsia lineatella in almonds in Colusa County, 1991.
Peach/nectarine results
Random shoot samples for PTB indicated that first PTB larval emergence began March 9, 8 days after the first treatment date in the Kingsburg peach and nectarine plot.
All treatment timings, except a single application at the end of petal fall, resulted in significantly fewer (P <0.01) shoot strikes than the untreated check (fig. 1). The single treatment at end-of-petal-fall, the jacket treatment, the two treatments at the beginning and end of petal fall, and the single pink-bud treatment were not significantly different than the single-jacket or end-of-petal-fall treatments (fig. 1). All the remaining treatments were significantly better than the two single treatments at the end-of-petal-fall and jacket stage. The best single treatment timing as measured by the least number of shoot strikes was the one applied at early (20%) bloom (fig. 1). The best overall treatment timing was two applications, at pink-bud and the beginning of petal fall. These and all treatment timings with three, four or five applications were not significantly different (P>0.01) from one another or from the standard organophosphate-plus-oil treatment. All were significantly (P <0.01) different from the untreated check.
Almond results
Our results for almonds in 1990 showed most single application timings, as measured by PTB shoot strikes (fig. 2), were not significantly different (P >0.05) than the untreated checks. However, significantly fewer strikes were observed when four Bt sprays were applied and compared with the standard organophosphate-plus-oil dormant treatment.
In 1991, our results indicated that several timings could significantly lower the number of shoot strikes compared with an untreated control. The best treatment comprised three Bt applications: at popcorn, petal fall and petal fall-plus-2-weeks (fig. 3). Two sprays applied 2 weeks apart at popcorn and petal fall and at petal fall alone were not significantly different (P >0.05) from that of the diazinon-plus-oil treatment. The other single-treatment timings had significantly greater (P <0.05) shoot strikes than the standard dormant spray (fig. 3).
Discussion
Although the 1990 peach and nectarine orchard had not received insecticide treatments for 6 years, PTB abundance would be considered “moderate.” At this population level, one application of Bt at 20% bloom or multiple applications before petal fall provided control of PTB equal to a dormant Diazinon-plus-oil application. Multiple Bt treatments in almonds in both 1990 and 1991 provided control equivalent to the standard dormant spray. Conditions in 1990 resulted in a “compact” bloom of short duration with little rainfall in both stone fruit and almond trials. In 1991, the almond bloom period was protracted, and the trial orchard received 12 inches of precipitation during the treatment period. In spite of these more challenging conditions, Bt treatments at popcorn and petal fall provided comparable control to the standard dormant treatment in 1991.
Our data indicate that it may be possible for growers to substitute well-timed Bt treatments during bloom for early season control of PTB. Used in conjunction with mating disruption for Oriental fruit moth, there is a good possibility that peaches, nectarines and prunes can be grown most years without using broad-spectrum insecticides. At minimum, this provides growers the option of avoiding dormant sprays in environmentally sensitive areas. Used in conjunction with winter sanitation and early harvest for navel orangeworm control, broad-spectrum insecticides can also be greatly reduced in almonds. In orchards where naval orangeworm parasites are present, this may increase their effectiveness because they would not be destroyed by dormant organophosphate sprays.
Two applications of Bt for PTB control are somewhat more expensive than a single organophosphate treatment, especially if application costs are considered. However, Bt is compatible with most fungicides and can be combined with brown rot treatments, applied at pink bud and full bloom, or with shot hole treatments, applied at petal fall to reduce application costs.
Although results look promising, we consider them preliminary. Research in large plots is under way to verify these results, to gain experience with the use of this material on a large scale and to answer many other questions relating to outbreaks of secondary pests. Growers interested in testing this approach should use it in moderation the first year and determine its suitability for their orchards.